Abstract
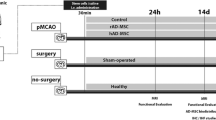
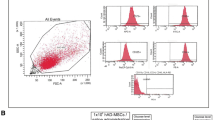
We aimed to evaluate whether adipose-derived mesenchymal stem cells (ADMSCs) that were transplanted via internal carotid can improve the neurological function after acute ischemic stroke and explore the underlying mechanisms. Total 40 adult Sprague–Dawley rats were subjected to transient (1.5 h) middle cerebral artery occlusion (MCAo) to induce ischemia/reperfusion injury. These rats were randomly divided into two groups with 20 ones in each group, which were intracarotid-injected with autologous ADMSCs (2.0 × 106) and saline (control) at day 3 after MCAo, respectively. Behavioral tests (adhesive-removal and modified neurological severity score) were performed before and after MCAo. Histology was used to evaluate the ischemia lesion volume and pathological changes. The apoptosis and astroglial reactivity were determined by TUNEL and glial fibrillary acidic protein (GFAP) staining, respectively. Besides, we applied immunofluorescence to identify the distribution of ADMSCs and the neural makers (NeuN and GFAP) expressed by them under confocal microscope. Significant improvement of neurological deficits was observed in rats transplanted with ADMSCs when compared to controls. But there was no obvious difference on ischemia lesion volume between these two groups. The injected ADMSCs migrated to the brain infarct region and mainly localized in the ischemic core and boundary zone of the lesion, which can express NeuN and GFAP in the brain. In addition, autologous transplantation of ADMSCs significantly attenuated astroglial reactivity, inhibited cellular apoptosis and promoted cellular proliferation. Our data indicated that intracarotid transplantation of autologous ADMSCs had the potential therapeutic application for ischemic stroke.







Similar content being viewed by others
References
Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53.
Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2009;7(4):CD000213.
Bravata DM. Intravenous thrombolysis in acute ischaemic stroke. CNS Drugs. 2005;19(4):295–302.
Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55(3):363–89.
Goldstein LB, Rothwell PM. Advances in prevention and health services delivery 2009. Stroke. 2010;41(2):e71–3.
Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265(1):73–7.
Abbott JD, Giordano FJ. Stem cells and cardiovascular disease. J Nucl Cardiol. 2003;10(4):403–12.
Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–70.
Suzuki K, Oyama M, Faulcon L, Robbins P, Niyibizi C. In vivo expression of human growth hormone by genetically modified murine bone marrow stromal cells and its effect on the cells in vitro. Cell Transpl. 2000;9(3):319.
Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu Y-X, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20(9):1311–9.
Zhao L-R, Duan W-M, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174(1):11–20.
Pansky A, Roitzheim B, Tobiasch E. Differentiation potential of adult human mesenchymal stem cells. Clin Lab. 2007;53(1–2):81.
Safford KM, Hicok KC, Safford SD, Halvorsen Y-DC, Wilkison WO, Gimble JM, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294(2):371–9.
Fujimura J, Ogawa R, Mizuno H, Fukunaga Y, Suzuki H. Neural differentiation of adipose-derived stem cells isolated from GFP transgenic mice. Biochem Biophys Res Commun. 2005;333(1):116–21.
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183(2):355–66.
Leu S, Lin Y-C, Yuen C-M, Yen C-H, Kao Y-H, Sun C-K, et al. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Translat Med. 2010;8(1):63.
Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666–72.
Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18(8):813–9.
Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83(11):433–7.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–11.
Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229.
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A Semiautomated Method for Measuring BrainInfarct Volume. J Cereb Blood Flow Metab. 1990;10(2):290–3.
Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45(4):514–22.
Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2006;27(1):6–13.
Mckeon RJ, Höke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136(1):32–43.
Pavlichenko N, Sokolova I, Vijde S, Shvedova E, Alexandrov G, Krouglyakov P, et al. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–13.
Ginsberg MD, Pulsinelli WA. The ischemic penumbra, injury thresholds, and the therapeutic window for acute stroke. Annals of neurology. 1994;36(4):553–4.
Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39(8):2362–9.
Li D, Fang Y, Wang P, Shan W, Zuo Z, Xie L. Autologous transplantation of adipose-derived mesenchymal stem cells attenuates cerebral ischemia and reperfusion injury through suppressing apoptosis and inducible nitric oxide synthase. Int J Mol Med. 2012;29(5):848.
Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297(5585):1299.
Acknowledgments
This study was supported by Social development research plan of Liaoning Province (2013225089) and National Natural Science Foundation of China (81300990).
Conflict of interest
All authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, W., Liang, G., Li, X. et al. Intracarotid transplantation of autologous adipose-derived mesenchymal stem cells significantly improves neurological deficits in rats after MCAo. J Mater Sci: Mater Med 25, 1357–1366 (2014). https://doi.org/10.1007/s10856-014-5157-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5157-9