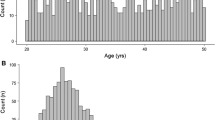
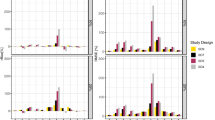
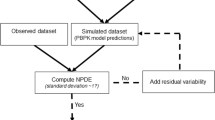
Abstract
To evaluate by simulation the statistical properties of normalized prediction distribution errors (NPDE), prediction discrepancies (pd), standardized prediction errors (SPE), numerical predictive check (NPC) and decorrelated NPC (NPCdec) for the external evaluation of a population pharmacokinetic analysis, and to illustrate the use of NPDE for the evaluation of covariate models. We assume that a model MB has been built using a building dataset B, and that a separate validation dataset, V is available. Our null hypothesis H0 is that the data in V can be described by MB. We use several methods to test this hypothesis: NPDE, pd, SPE, NPC and NPCdec. First, we evaluated by simulation the type I error under H0 of different tests applied to the four methods. We also propose and evaluate a single global test combining normality, mean and variance tests applied to NPDE, pd and SPE. We perform tests on NPC and NPCdec, after a decorrelation. MB was a one compartment model with first order absorption (without covariate), previously developed from two phase II and one phase III studies of the antidiabetic drug, gliclazide. We simulated 500 external datasets according to the design of a phase III study. Second, we investigated the application of NPDE to covariate models. We propose two approaches: the first approach uses correlation tests or mean comparisons to test the relationship between NPDE and covariates; the second evaluates NPDE split by category for discrete covariates or quantiles for continuous covariates. We generated several validation datasets under H0 and under alternative assumptions with a model without covariate, with one continuous covariate (weight), or one categorical covariate (sex). We calculated the powers of the different tests using simulations, where the covariates of the phase III study were used. The simulations under H0 show a high type I error for the different tests applied to SPE and an increased type I error for pd. The different tests present a type I error close to 5% for the global test appied to NPDE. We find a type I error higher than 5% for the test applied to classical NPC but this test becomes close to 5% for NPCdec. For covariate models, when model and validation dataset are consistent, type I error of the tests are close to 5% for both effects. When validation datasets and models are not consistent, the tests detect the correlation between NPDE and the covariate. We recommend to use NPDE over SPE for external model evaluation, since they do not depend on an approximation of the model and have good statistical properties. NPDE represent a better approach than NPC, since in order to perform tests on NPC, a decorrelation step must be applied before. NPDE, in this illustration, is also a good tool to evaluate model with or without covariates.





Similar content being viewed by others
References
Powell JR, Gobburu JV (2007) Pharmacometrics at FDA: evolution and impact on decisions. Clin Pharmacol Ther 82(1):97–102. Epub 2007 May 30
Wade JR, Edholm M, Salmonson T (2005) A guide for reporting the results of population pharmacokinetic analyses: a Swedish perspective. Aaps J 7(2):45
Karlsson MO, Holford NH (2008) A tutorial on visual predictive checks. p 17. Abstr 1434 [www.page-meeting.org/?abstract=1434]
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B et al (2007) Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet 46(3):221–234
Mentre F, Escolano S (2006) Prediction discrepancies for the evaluation of nonlinear mixed-effects models. J Pharmacokinet Pharmacodyn 33(3):345–367. Epub 2005 Nov 13
Brendel K, Comets E, Laffont C, Laveille C, Mentre F (2006) Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23(9):2036–2049. Epub 2006 Aug 12
Holford NH (2005) The visual Predictive Check-Superiority to standard diagnostic (Rorschach) plots. p 14. Abstr 738 [www.page-meeting.org/?abstract=738]
Wilkins J, Karlsson M, Jonsson EN (2006) Patterns and power for the visual predictive check. p 15. Abstr 1029 [www.page-meeting.org/?abstract=1029]
Frey N, Laveille C, Paraire M, Francillard M, Holford NH, Jochemsen R (2003) Population PKPD modelling of the long-term hypoglycaemic effect of gliclazide given as a once-a-day modified release (MR) formulation. Br J Clin Pharmacol 55(2):147–157
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
Beal SL, Sheiner LB (1992) NONMEM users guides. NONMEM Project Group Ed. University of California, San Francisco
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24(12):2187–2197. Epub 2007 Jul 6
Post TM, Freijer JI, Ploeger BA, Danhof M (2008) Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn 35(2):185–202. Epub 2008 Jan 16
Samson A, Lavieille M, Mentré F (2006) Extension of the SAEM algorithm to left-censored data in nonlinearmixed-effects model: application to HIV dynamics model. Comput Stat Data Anal 51:1562–1574
Brendel K, Comets E, Laffont C, Lemenuel-Diot A, Mentré F (2006) Normalized prediction distribution errors for the evaluation of a population pharmacodynamic model for gliclazide. PKPD Congress, Leiden
Comets E, Brendel K, Mentre F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90(2):154–166. Epub 2008 Jan 22
Lavieille M (2005) MONOLIX (Modèles NOn LInéaires à effets miXtes)., 2005MONOLIX group, Orsay, France
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brendel, K., Comets, E., Laffont, C. et al. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn 37, 49–65 (2010). https://doi.org/10.1007/s10928-009-9143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-009-9143-7