Abstract
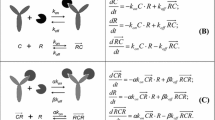
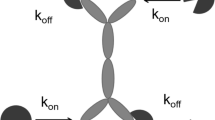
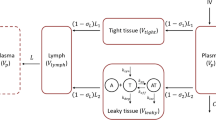
Until recently, most therapeutic monoclonal antibodies (mAb) were designed to bind only one target. However, several existing mAbs bind to soluble and membrane forms of the same receptor. Moreover, design of bi-specific and multi-specific proteins that bind to more than one target is a promising direction of drug design. The pharmacokinetics and pharmacodynamics of these drugs may be described by the target-mediated drug disposition (TMDD). This work extended the TMDD model to drugs that bind more than one target. The quasi-steady-state (QSS) and Michaelis–Menten (MM) approximations of the model were also derived. Identifiability of model parameters was studied by simulations. The drug and target parameters used in simulations were chosen to imitate a monoclonal antibody that binds to the soluble (S) and membrane-bound (M) targets. The data were simulated for 224 subjects using the full TMDD model and dosing that mimicked typical Phase I and Phase II designs with rich sampling. Four population pharmacokinetic models were fitted to the free (unbound) drug and total (unbound and bound to the drug) S-target data: a one-target QSS model that simultaneously described the free drug and the total S-target (M1), a model with parallel linear and MM elimination that described the free drug combined with a separate S-target model that utilized the free drug concentrations but did not influence them (M2), a two-target QSS model where the S-target was described by the QSS approximation while the contribution of the M-target was described by the MM elimination term (M3), and a two-target full TMDD model (M4). The influence of relative contributions of the S and M-targets to target-mediated elimination on identifiability of the model parameters was investigated. The influence of assay sensitivity and availability of the total rather than free drug concentration measurements were also investigated. The results indicated that for the dosing regimens and system parameters investigated in this work the pharmacokinetic data alone did not allow to distinguish influences of the two targets. When the drug and S-target data were available, the model M1 described the data with the deficiencies of the fit visible only at the lowest dose level. However, the parameter estimates were strongly biased. The model M2 improved the fit and provided the precise estimates of the S-target parameters. However, no information concerning the M-target could be obtained from this model. The model M3 provided an excellent description of the data and the unbiased estimates of all the parameters. It also provided the unbiased estimates of change from baseline of the unobservable M-target concentrations. The models M1-M3 were robust while M4 was unstable despite the prohibitively long run time. The results were similar when the total rather than free drug was measured. The M-target parameters were estimated only when M-target elimination was at least comparable to S-target elimination. Improvement of the assay sensitivity has not resulted in marked improvement of the parameter estimates. In summary, for the cases investigated in this work the QSS approximation of the two-target TMDD model provided the unbiased and robust estimates of all the relevant TMDD parameters.







Similar content being viewed by others
References
Levy G (1994) Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 56:248–252
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Mager DE, Krzyzanski W (2005) Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res 22(10):1589–1596
Gibiansky L, Gibiansky E, Kakkar T, Ma P (2008) Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn 35(5):573–591
Gibiansky L, Gibiansky E (2009) Target-mediated drug disposition model: approximations, identifiability of model parameters, and applications to the population pharmacokinetic–pharmacodynamic modeling of biologics. Expert Opin Drug Metabol Toxicol 5(7):803–812
Gibiansky L, Gibiansky E (2009) Target-mediated drug disposition model: relationships with indirect response models and application to population PK-PD analysis. J Pharmacokinet Pharmacodyn 36(4):341–351
Marathe A, Krzyzanski W, Mager DE (2009) Numerical validation and properties of a rapid binding approximation of a target-mediated drug disposition pharmacokinetic model. J Pharmacokinet Pharmacodyn 36(3):199–219
Yan X, Mager DE, Krzyzanski W (2010) Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn 37(1):25–47
Peletier LA, Gabrielsson J (2009) Dynamics of target-mediated drug disposition. Eur J Pharm Sci 38(5):445–464
Grimm HP (2009) Gaining insights into the consequences of target-mediated drug disposition of monoclonal antibodies using quasi-steady-state approximations. J Pharmacokinet Pharmacodyn 36(5):407–420
Krippendorff BF, Kuester K, Kloft C, Huisinga W (2009) Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn 36(3):239–260
Lees KR, Kelman AW, Reid JL, Whiting B (1989) Pharmacokinetics of and ACE inhibitor, S-9780, in men: evidence of tissue Binding. J Pharmacokinet Pharmacodyn 17(5):529–550
Tannenbaum S, Gautier A, Lowe P (2008) A mechanism based binding model for the population pharmacokinetics and pharmacodynamics of canakinumab, a monoclonal antibody in development for rheumatoid arthritis. AAPS J 10(S2). www.aapsj.org/abstracts/AM_2008/AAPS2008-002581.PDF
Retlich S, Duval V, Graefe-Mody U, Jaehde U, Staab A (2010) Impact of target-mediated drug disposition on linagliptin pharmacokinetics and dpp-4 inhibition in type 2 diabetic patients. J Clin Pharmacol. doi:10.1177/0091270009356444
Snoeck E, Jacqmin P, Van Peer A, Danhof M (2009) A combined specific target site binding and pharmacokinetic model to explore the non-linear disposition of draflazine. J Pharmacokinet Biopharm 27(3):257–281
Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, Fuh G (2009) Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 323(5921):1610–1614
European Medicines Agency (EMEA) (2008) European public assessment report—RoActemra. EMEA website
Meulen CG, Göertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, Hoitsma AJ (2003) Decreased renal excretion of soluble interleukin-2 receptor alpha after treatment with daclizumab. Kidney Int 64(2):697–703
Nestorov I, Munafo A, Papasouliotis O, Visich J (2008) Pharmacokinetics and biological activity of atacicept in patients with rheumatoid arthritis. J Clin Pharmacol 48:406–417
Kuester K, Kloft C (2006) Pharmacokinetics of monoclonal antibodies. In: Meibohm B (ed) Pharmacokinetics and pharmacodynamics of biotech drugs: principles and case studies in drug development. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 45–91
Mould DR, Sweeney KD (2007) A review of the pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modeling applied to drug development. Current Opin Drug Discov Dev 10(1):84–96
Wand W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84(5):548–558
Moeniralam HS, Bemelman WA, Endert E, Koopmans R, Sauerwein HP, Romijn JA (1997) The decrease in nonsplenic interleukin-6 (IL-6) production after splenectomy indicates the existence of a positive feedback loop of IL-6 production during endotoxemia in dogs. Infect Immun 65(6):2299–2305
Lu ZY, Brailly H, Wijdenes J, Bataille R, Rossi JF, Klein B (1995) Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments. Blood 86(8):3123–3131
Taguchi T (1988) Phase I study of recombinant human tumor necrosis factor (rHu-TNF:PT-050). Cancer Detect Prev 12(1–6):561–572
Moritz T, Niederle N, Baumann J, May D, Kurschel E, Osieka R, Kempeni J, Schlick E, Schmidt CG (1989) Phase I study of recombinant human tumor necrosis factor alpha in advanced malignant disease. Cancer Immunol Immunother 29(2):144–145
Wargalla UC, Reisfeld RA (1989) Rate of internalization of an immunotoxin correlates with cytotoxic activity against human tumor cells. Proc Natl Acad Sci USA 86:5146–5150
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ (1989–2009) NONMEM user’s guides. Icon Development Solutions, Ellicott City
Kakkar T, Gibiansky L, Ma P (2009) Population pharmacokinetics of AMG 317, a fully human anti-IL-4Rα IgG2 monoclonal antibody evaluated in healthy and asthmatic subjects. AAPS J 11(S2). www.aapsj.org/abstracts/AM_2009/AAPS2009-002216.PDF
Hayashi N, Tsukamoto Y, Sallas WM, Lowe PJ (2007) A mechanism-based binding model for the population pharmacokinetics and pharmacodynamics of omalizumab. Br J Clin Pharmacol 63(5):548–561
Tannenbaum S, Chakraborty A, Rordorf C, Gautier A, Lowe P (2009) Pharmacokinetics of canakinumab and pharmacodynamics of IL-1β binding in patients with cryopyrin associated periodic fever syndromes, Abstr 1592. p 8. www.page-meeting.org/?abstract=1592
Vu T, Lee E, Narayanan N, Jang G, Ma P (2010) Predicting free sclerostin from free AMG 785 and total sclerostin, Abstr 1939. p 19. www.page-meeting.org/?abstract=1939
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibiansky, L., Gibiansky, E. Target-mediated drug disposition model for drugs that bind to more than one target. J Pharmacokinet Pharmacodyn 37, 323–346 (2010). https://doi.org/10.1007/s10928-010-9163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-010-9163-3