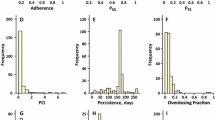
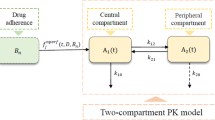
Abstract
Poor adherence to a drug prescription significantly impacts on the efficacy and safety of a planned therapy. The relationship between drug intake and pharmacokinetics (PK) is only partially known. In this work, we focus on the so-called “inverse problem”, concerned with the issue of retracing the patient compliance scenario using limited clinical knowledge. Using a reported Pop-PK model of imatinib, and accounting for the variability around its PK parameters, we were able to simulate a whole range of drug concentration values at a specific sampling point for a population of patients with all possible drug compliance profiles. Using a Bayesian decision rule, we developed a methodology for the determination of the associated compliance profile prior to a given sampling value. The adopted approach allows, for the first time, to quantitatively acquire knowledge about the compliance patterns having a causal effect on a given PK. Moreover, using a simulation approach, we were able to evaluate the evolution of success rate of the retracing process in terms of the considered time period before sampling as well as the model-inherited variability. In conclusion, this work allows, from a probability viewpoint, to propose a solution for this inverse problem of compliance determination.


















Similar content being viewed by others
References
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M (2008) Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 336(7653):1114–1119
Urquhart J (2000) Erratic patient compliance with prescribed drug regimens: target for drug. Clin Pharmacol Ther 67(4):331–334
Hénin E, Tod M, Trillet-Lenoir V, Rioufol C, Tranchand B, Girard P (2009) Pharmacokinetically based estimation of patient compliance with oral anticancer chemotherapies: in silico evaluation. Clin Pharmacokinet 48(6):359–369
Li J, Nekka F (2007) A pharmacokinetic formalism explicitly integrating the patient drug compliance. J Pharmacokinet Pharmacodyn 34(1):115–139
Mu S, Ludden TM (2003) Estimation of population harmacokinetic parameters in the presence of non-compliance. JPKPD 30(1):53–81
Soy D, Beal SL, Sheiner LB (2004) Population one-compartment pharmacokinetic analysis with missing dosage data. Clin Pharmacol Ther 76(5):441–451
Jonsson EN, Wade JR, Almqvist G et al (1997) Discrimination between rival dosing histories. Pharm Res 14(8):984–991
Girard P, Sheiner L, Kastrissios H, Blaschke T (1996) Do we need full compliance data for population pharmacokinetic analysis?. J Pharmacokinet Biopharm 24(3):265–282
Vrijens B, Goetghebeur E (2004) Electronic monitoring of variation in drug intakes can reduce bias and improve precision in pharmacokinetic/pharmacodynamic population studies. Stat Med 23(4):531–544
Li J, Nekka F (2009) A probabilistic approach for the evaluation of pharmacological effect induced by patient irregular drug intake. J Pharmacokinet Pharmacodyn 36(3):221–246
Widmer N, Decosterd LA, Csajka C, Leyvraz S, Duchosal MA, Rosselet A, Rochat B, Eap CB, Henry H, Biollaz J, Buclin T (2006) Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol 62(1):97–112
Duda RO, Hart PE, Stork DG (2001) Pattern classification, 2nd edn. Wiley, New York
Acknowledgment
This work has been supported by FQRNT, NSERC and MITACS. The Centre de Recherches Mathématiques of Université de Montréal is also acknowledged for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrière, O., Li, J. & Nekka, F. A Bayesian approach for the estimation of patient compliance based on the last sampling information. J Pharmacokinet Pharmacodyn 38, 333–351 (2011). https://doi.org/10.1007/s10928-011-9196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-011-9196-2