Abstract
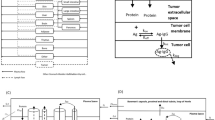
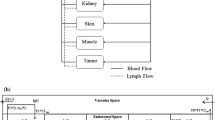
In this work we proposed a population physiologically-based pharmacokinetic (popPBPK) framework for quantifying and predicting inter-individual pharmacokinetic variability using the anti-HER2 monoclonal antibody (mAb) trastuzumab as an example. First, a PBPK model was developed to account for the possible mechanistic sources of variability. Within the model, five key factors that contribute to variability were identified and the nature of their contribution was quantified with local and global sensitivity analyses. The five key factors were the concentration of membrane-bound HER2 (\(Ag\)), the convective flow rate of mAb through vascular pores (\(F2\)), the endocytic transport rate of mAb through vascular endothelium (\(CL_{up}\)), the degradation rate of mAb-HER2 complexes (\(K_{deg}^{Ag}\)) and the concentration of shed HER2 extracellular domain in circulation (\(ECD\)). \(F2\) was the most important parameter governing trastuzumab distribution into tissues and primarily affected variability in the first 500 h post-administration. \(Ag\) was the most significant contributor to variability in clearance. These findings were used together with population generation methods to accurately predict the observed variability in four experimental trials with trastuzumab. To explore anthropometric sources of variability, virtual populations were created to represent participants in the four experimental trials. Using populations with only their expected anthropometric diversity resulted in under-prediction of the observed inter-individual variability. Adapting the populations to include literature-based variability around the five key parameters enabled accurate predictions of the variability in the four trials. The successful application of this framework demonstrates the utility of popPBPK methods to understand the mechanistic underpinnings of pharmacokinetic variability.











Similar content being viewed by others
References
Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P (2005) Population pharmacokinetics of trastuzumab in patients with HER2 + metastatic breast cancer. Cancer Chemother Pharmacol 56(4):361–369. doi:10.1007/s00280-005-1026-z
Schmidt H (2013) SBPOP Package: efficient support for model based drug development—from mechanistic models to complex trial simulation. PAGE meeting, Glasgow
Willmann S, Höhn K, Edginton A, Sevestre M, Solodenko J, Weiss W, Lippert J, Schmitt W (2007) Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn 34(3):401–431. doi:10.1007/s10928-007-9053-5
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39(1):67–86. doi:10.1007/s10928-011-9232-2
Cao Y, Jusko WJ (2014) Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn 41(4):375–387. doi:10.1007/s10928-014-9372-2
Li L, Gardner I, Rose R, Jamei M (2014) Incorporating target shedding into a minimal PBPK-TMDD model for monoclonal antibodies. CPT Pharmacomet Syst Pharmacol 3:e96. doi:10.1038/psp.2013.73
Glassman PM, Balthasar JP (2016) Physiologically-based pharmacokinetic modeling to predict the clinical pharmacokinetics of monoclonal antibodies. J Pharmacokinet Pharmacodyn 43(4):427–446. doi:10.1007/s10928-016-9482-0
Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D (2003) Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem 278(26):23343–23351. doi:10.1074/jbc.M300477200
Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D (2003) Coregulation of epidermal growth factor receptor/human epidermal growth factor receptor 2 (HER2) levels and locations: quantitative analysis of HER2 overexpression effects. Cancer Res 63(5):1130–1137
Shankaran H, Wiley HS, Resat H (2006) Modeling the effects of HER/ErbB1-3 coexpression on receptor dimerization and biological response. Biophys J 90(11):3993–4009. doi:10.1529/biophysj.105.080580
Resat H, Ewald JA, Dixon DA, Wiley HS (2003) An integrated model of epidermal growth factor receptor trafficking and signal transduction. Biophys J 85(2):730–743
Valabrega G, Montemurro F, Sarotto I, Petrelli A, Rubini P, Tacchetti C, Aglietta M, Comoglio PM, Giordano S (2005) TGFalpha expression impairs trastuzumab-induced HER2 downregulation. Oncogene 24(18):3002–3010. doi:10.1038/sj.onc.1208478
Stancovski I, Hurwitz E, Leitner O, Ullrich A, Yarden Y, Sela M (1991) Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci USA 88(19):8691–8695
Longva KE, Pedersen NM, Haslekas C, Stang E, Madshus IH (2005) Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer 116(3):359–367. doi:10.1002/ijc.21015
Zhu W, Okollie B, Artemov D (2007) Controlled internalization of Her-2/neu receptors by cross-linking for targeted delivery. Cancer Biol Ther 6(12):1960–1966
Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH (2004) Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell 15(12):5268–5282. doi:10.1091/mbc.E04-07-0591
Gill KL, Gardner I, Li L, Jamei M (2016) A bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J 18(1):156–170. doi:10.1208/s12248-015-9819-4
Huwaldt J (2015) Plot Digitizer
Morita J, Tanaka M, Nomoto M, Matsuki S, Tsuru T, Matsuguma K, Shiramoto M (2016) Pharmacokinetic bioequivalence, safety, and immunogenicity of DMB-3111, a trastuzumab biosimilar, and trastuzumab in healthy japanese adult males: results of a randomized trial. BioDrugs 30(1):17–25. doi:10.1007/s40259-015-0153-2
Wisman LA, De Cock EP, Reijers JA, Kamerling IM, Van Os SH, de Kam ML, Burggraaf J, Voortman G (2014) A phase I dose-escalation and bioequivalence study of a trastuzumab biosimilar in healthy male volunteers. Clin Drug Investig 34(12):887–894. doi:10.1007/s40261-014-0247-5
Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B (2013) Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol 53(2):192–201. doi:10.1177/0091270012436560
Yin D, Barker KB, Li R, Meng X, Reich SD, Ricart AD, Rudin D, Taylor CT, Zacharchuk CM, Hansson AG (2014) A randomized phase 1 pharmacokinetic trial comparing the potential biosimilar PF-05280014 with trastuzumab in healthy volunteers (REFLECTIONS B327-01). Br J Clin Pharmacol 78(6):1281–1290. doi:10.1111/bcp.12464
Tokuda Y, Watanabe T, Omuro Y, Ando M, Katsumata N, Okumura A, Ohta M, Fujii H, Sasaki Y, Niwa T, Tajima T (1999) Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br J Cancer 81(8):1419–1425. doi:10.1038/sj.bjc.6690343
Baselga J, Carbonell X, Castaneda-Soto NJ, Clemens M, Green M, Harvey V, Morales S, Barton C, Ghahramani P (2005) Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol 23(10):2162–2171. doi:10.1200/JCO.2005.01.014
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Leyland-Jones B, Gelmon K, Ayoub JP, Arnold A, Verma S, Dias R, Ghahramani P (2003) Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 21(21):3965–3971. doi:10.1200/JCO.2003.12.109
Di Gioia D, Dresse M, Mayr D, Nagel D, Heinemann V, Kahlert S, Stieber P (2014) Serum HER2 supports HER2-testing in tissue at the time of primary diagnosis of breast cancer. Clin Chim Acta 430:86–91. doi:10.1016/j.cca.2013.12.036
Lam L, Czerniecki BJ, Fitzpatrick E, Xu S, Schuchter L, Xu X, Zhang H (2014) Interference-free HER2 ECD as a serum biomarker in breast cancer. J Mol Biomark Diagn 4(3):151. doi:10.4172/2155-9929.1000151
McCarthy KM, Lam M, Subramanian L, Shakya R, Wu Z, Newton EE, Simister NE (2001) Effects of mutations in potential phosphorylation sites on transcytosis of FcRn. J Cell Sci 114(Pt 8):1591–1598
McCarthy KM, Yoong Y, Simister NE (2000) Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J Cell Sci 113(Pt 7):1277–1285
Parving HH, Jensen HA, Westrup M (1977) Increased transcapillary escape rate of albumin and IgG in essential hypertension. Scand J Clin Lab Invest 37(3):223–227. doi:10.3109/00365517709091486
Gill KL, Machavaram KK, Rose RH, Chetty M (2016) Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet 55(7):789–805. doi:10.1007/s40262-015-0361-4
Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T (2010) Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol 184(4):1968–1976. doi:10.4049/jimmunol.0903296
Li L, Gardner I, Dostalek M, Jamei M (2014) Simulation of monoclonal antibody pharmacokinetics in humans using a minimal physiologically based model. AAPS J 16(5):1097–1109. doi:10.1208/s12248-014-9640-5
Lua W-H, Gan SK-E, Lane DP, Verma CS (2015) A search for synergy in the binding kinetics of Trastuzumab and Pertuzumab whole and F(ab) to Her2. Npj Breast Cancer 1:15012. doi:10.1038/npjbcancer.2015.12
Troise F, Cafaro V, Giancola C, D’Alessio G, De Lorenzo C (2008) Differential binding of human immunoagents and Herceptin to the ErbB2 receptor. FEBS J 275(20):4967–4979. doi:10.1111/j.1742-4658.2008.06625.x
Larson JS, Goodman LJ, Tan Y, Defazio-Eli L, Paquet AC, Cook JW, Rivera A, Frankson K, Bose J, Chen L, Cheung J, Shi Y, Irwin S, Kiss LD, Huang W, Utter S, Sherwood T, Bates M, Weidler J, Parry G, Winslow J, Petropoulos CJ, Whitcomb JM (2010) Analytical validation of a highly quantitative, sensitive, accurate, and reproducible assay (HERmark) for the measurement of HER2 total protein and HER2 homodimers in FFPE breast cancer tumor specimens. Pathol Res Int 2010:814176. doi:10.4061/2010/814176
Huang W, Reinholz M, Weidler J, Yolanda L, Paquet A, Whitcomb J, Lingle W, Jenkins RB, Chen B, Larson JS, Tan Y, Sherwood T, Bates M, Perez EA (2010) Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. Am J Clin Pathol 134(2):303–311. doi:10.1309/AJCP3BZY4YAFNTRG
Olsen DA, Ostergaard B, Bokmand S, Wamberg PA, Jakobsen EH, Jakobsen A, Brandslund I (2009) HER1-4 protein concentrations in normal breast tissue from breast cancer patients are expressed by the same profile as in the malignant tissue. Clin Chem Lab Med 47(8):977–984. doi:10.1515/CCLM.2009.214
Weigle WO (1958) Elimination of antigen-antibody complexes from sera of rabbits. J Immunol 81(3):204–213
Berson SA, Yalow RS (1959) Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest 38:1996–2016. doi:10.1172/JCI103979
Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S (2013) An estimation of the number of cells in the human body. Ann Hum Biol 40(6):463–471. doi:10.3109/03014460.2013.807878
Cho H-S, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Leahy DJ (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421(6924):756–760
Saltelli A, Annoni P, Azzini I, Campolongo F, Ratto M, Tarantola S (2010) Variance based sensitivity analysis of model output. Design and estimator for the total sensitivity index. Comput Phys Commun 181(2):259–270. doi:10.1016/j.cpc.2009.09.018
Jacquez JA, Greif P (1985) Numerical parameter identifiability and estimability: integrating identifiability, estimability, and optimal sampling design. Math Biosci 77(1):201–227. doi:10.1016/0025-5564(85)90098-7
Parving HH (1976) Increased microvascular permeability to plasma proteins in short- and long-term juvenile diabetics. Diabetes 25(2 SUPPL):884–889
Parving HH, Rossing N (1973) Simultaneous determination of the transcapillary escape rate of albumin and IgG in normal and long-term juvenile diabetic subjects. Scand J Clin Lab Invest 32(3):239–244
Parving HH, Ranek L, Lassen NA (1977) Increased transcapillary escape rate of albumin in patients with cirrhosis of the liver. Scand J Clin Lab Invest 37(7):643–648
Hesse B, Parving HH, Lund-Jacobsen H, Noer I (1976) Transcapillary escape rate of albumin and right atrial pressure in chronic congestive heart failure before and after treatment. Circ Res 39(3):358–362
Parving HH, Worm AM, Rossing N (1976) Plasma volume, intravascular albumin and its transcapillary escape rate in patients with extensive skin disease. Br J Dermatol 95(5):519–524
Leung HWC, Chan ALF (2015) Trastuzumab-induced cardiotoxicity in elderly women with HER-2-positive breast cancer: a meta-analysis of real-world data. Expert Opin Drug Saf 14(11):1661–1671. doi:10.1517/14740338.2015.1089231
Carney WP, Neumann R, Lipton A, Leitzel K, Ali S, Price CP (2003) Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin Chem 49(10):1579–1598
Carney WP (2007) Circulating oncoproteins HER2/neu, EGFR and CAIX (MN) as novel cancer biomarkers. Expert Rev Mol Diagn 7(3):309–319. doi:10.1586/14737159.7.3.309
Ghedini GC, Ciravolo V, Tortoreto M, Giuffre S, Bianchi F, Campiglio M, Mortarino M, Figini M, Coliva A, Carcangiu ML, Zambetti M, Piazza T, Ferrini S, Menard S, Tagliabue E, Pupa SM (2010) Shed HER2 extracellular domain in HER2-mediated tumor growth and in trastuzumab susceptibility. J Cell Physiol 225(1):256–265. doi:10.1002/jcp.22257
Esteva FJ, Cheli CD, Fritsche H, Fornier M, Slamon D, Thiel RP, Luftner D, Ghani F (2005) Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res 7(4):R436–443. doi:10.1186/bcr1020
Tse C, Brault D, Gligorov J, Antoine M, Neumann R, Lotz JP, Capeau J (2005) Evaluation of the quantitative analytical methods real-time PCR for HER-2 gene quantification and ELISA of serum HER-2 protein and comparison with fluorescence in situ hybridization and immunohistochemistry for determining HER-2 status in breast cancer patients. Clin Chem 51(7):1093–1101. doi:10.1373/clinchem.2004.044305
Fornier MN, Seidman AD, Schwartz MK, Ghani F, Thiel R, Norton L, Hudis C (2005) Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann Oncol 16(2):234–239. doi:10.1093/annonc/mdi059
Kostler WJ, Schwab B, Singer CF, Neumann R, Rucklinger E, Brodowicz T, Tomek S, Niedermayr M, Hejna M, Steger GG, Krainer M, Wiltschke C, Zielinski CC (2004) Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res 10(5):1618–1624
Bethune-Volters A, Labroquere M, Guepratte S, Hacene K, Neumann R, Carney W, Pichon MF (2004) Longitudinal changes in serum HER-2/neu oncoprotein levels in trastuzumab-treated metastatic breast cancer patients. Anticancer Res 24(2C):1083–1089
Chetty M, Li L, Rose R, Machavaram K, Jamei M, Rostami-Hodjegan A, Gardner I (2014) Prediction of the pharmacokinetics, pharmacodynamics, and efficacy of a monoclonal antibody, using a physiologically based pharmacokinetic FcRn model. Front Immunol 5:670. doi:10.3389/fimmu.2014.00670
Chen Y, Balthasar JP (2012) Evaluation of a catenary PBPK model for predicting the in vivo disposition of mAbs engineered for high-affinity binding to FcRn. AAPS J 14(4):850–859. doi:10.1208/s12248-012-9395-9
Homma T, Saltelli A (1996) Importance measures in global sensitivity analysis of nonlinear models. Reliab Eng Syst Safe 52(1):1–17. doi:10.1016/0951-8320(96)00002-6
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malik, P.R.V., Hamadeh, A., Phipps, C. et al. Population PBPK modelling of trastuzumab: a framework for quantifying and predicting inter-individual variability. J Pharmacokinet Pharmacodyn 44, 277–290 (2017). https://doi.org/10.1007/s10928-017-9515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-017-9515-3