Abstract
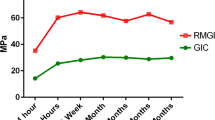
The thermal characteristics of four conventional glass-ionomer cement (GIC) dental restorative products as well as five resin-modified glass-ionomer (RMGI) materials over 1-year of storage were investigated. All materials were prepared following manufacturer’s recommendations and placed into 40 μL aluminum differential scanning calorimeter (DSC) crucibles. Samples (n = 5) were stored at 37 °C and 98 ± 2 % humidity until their appointed time of evaluation at which they were first subjected to specific heat analysis using DSC over 20–60 °C that was immediately followed by a 37–600 °C thermal scan at 10 °C min−1. Samples were evaluated immediately after preparation, at 24 h, 1 week, 1 month, as well as at 3, 6, 9, and 12 months. Mean thermal results were compared with analysis of variance and Scheffe post-hoc testing (p = 0.05). All materials absorbed water during storage. Conventional GIC materials demonstrated increased polyalkenoate polymer maturity over the 12-month storage. The paste–paste RMGI materials, absorbed more water during storage and had increased specific heat values compared to powder–liquid RMGI materials. Of the RMGI materials investigated, only two materials demonstrated evidence of a continuing polyalkenoate matrix maturity that was within the limitations of the technology used, indicating the resin component in some newer formulations of RMGI restorative materials may severely limit the polyalkenoate reaction.

Similar content being viewed by others
References
Wilson AD, Kent BE. The glass-ionomer cement, a new translucent dental filling material. J Appl Chem Biotechnol. 1971;21:313.
Wilson AD. Developments in glass-ionomer cements. Int J Prosthodont. 1989;2:438–46.
Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–94.
Prosser HJ, Powis DR, Brant P, Wilson AD. Characterization of glass-ionomer cements. 7. The physical properties of current materials. J Dent. 1984;12:231–40.
McLean JW, Gasser O. Glass-cermet cements. Quintessence Int. 1985;16:333–43.
Simmons JJ. The miracle mixture: glass-ionomer and alloy powder. Texas Dent J. 1983;100:6–12.
Williams JA, Billington RW, Pearson GJ. The comparative strengths of commercial glass-ionomer cements with and without metal additions. Br Dent J. 1992;172:279–82.
Guggenberger R, May R, Stefan KP. New trends in glass-ionomer chemistry. Biomaterials. 1998;19:479–83.
McLean JW, Nicholson JW, Wilson AD. Proposed nomenclature for glass-ionomer cements and related materials. Quintessence Int. 1994;25:587–9.
Sidhu SK, Watson TF. Resin-modified glass ionomer materials. A status report for the American Journal of Dentistry. Am J Dent. 1995;8:59–67.
Davidson CL, Mjör IA. Advances in glass-ionomer cements. Chicago: Quintessence Publishing Co., Inc.; 1999.
Wilson AD. Resin-modified glass-ionomers. Int J Prosthodont. 1990;3:425–9.
de Gee AJ, Leloup G, Werner A, Vreven J, Davidson CL. Structural integrity of resin-modified glass ionomers as affected by the delay or omission of light activation. J Dent Res. 1998;77:1658–63.
Hammesfahr PD (1994) Developments in resionomer systems. In: Hunt P, (ed) Glass ionomers: the next generation. Proceedings of the 2nd International Symposium on Glass Ionomers, June 1994. International Symposia in Dentistry, PC. Philadelphia, pp. 47–55.
Eliades G, Palaghias G. In vitro characterization of visible light-cured glass ionomers liners. Dent Mater. 1993;9:198–203.
Culbertson BM. Glass-ionomer dental restoratives. Prog Polym Sci. 2001;26:577–604.
Fuji II LC MSDS. 2000 http://www.gcamerica.com. Accessed 25 Feb 2012.
Vitremer and Photac-Fil Quick MSDS. 2012. http://www3.3m.com/search/ww/en001/msdssearchform.do. Accessed 25 Feb 2012.
Coutinho E, Cardoso MV, De Munck J, Neves AA, Van Landuyt KL, Poitevin A, Peumans M, Lambrechts P, Van Meerbeek B. Bonding effectiveness and interfacial characterization of a nano-filled resin-modified glass-ionomer. Dent Mater. 2009;25:1347–57.
Anstice HM, Nicholson JW. Studies in the setting of polyelectrolyte materials. Part II: the effect of organic compounds on a glass poly(alkenoate) cement. J Mater Sci Mater Med. 1994;5:299–302.
Nicholson JW, Anstice HM. The physical chemistry of light-curable glass-ionomers. J Mater Sci Mater Med. 1994;5:119–22.
Wan ACA, Yap AUJ, Hastings GW. Acid-base complex reactions in resin-modified and conventional glass ionomer cements. J Biomed Mater Res. 1999;48:700–4.
Young AM. FTIR investigation of polymerisation and polyacid neutralisation kinetics in resin-modified glass-ionomer dental cements. Biomaterials. 2002;23:3289–95.
Young AM, Rafeeka SA, Howlett JA. FTIR investigation of monomer polymerisation and polyacid neutralisation kinetics and mechanisms in various aesthetic dental restorative materials. Biomaterials. 2004;25:823–33.
Li J, von Beetzen M, Sundström F. Strength and setting behavior of resin-modified glass ionomers cements. Acta Odontol Scand. 1995;53:311–7.
Kakaboura A, Eliades G, Palaghias G. An FTIR study on the setting mechanism of resin-modified glass ionomer restoratives. Dent Mater. 1996;12:173–8.
Young AM, Sherpa A, Pearson G, Schottlander B, Waters DN. Use of Raman spectroscopy in the characterisation of the acid-base reaction in glass-ionomer cements. Biomaterials. 2000;21:1971–9.
Chedella SC, Berzins DW. A differential scanning calorimetry study of the setting reaction of MTA. Int Endod J. 2010;43:509–18.
Cadenaro M, Navarra CO, Antoniolli F, Mazzoni A, Di Lenarda R, Rueggeberg FA, Breschi L. The effect of curing mode on extent of polymerization and microhardness of dual-cured, self-adhesive resin cements. Am J Dent. 2010;23:14–8.
Pfeifer CS, Ferracane JL, Sakaguchi RL, Braga RR. Photoinitiator content in restorative composites: influence on degree of conversion, reaction kinetics, volumetric shrinkage and polymerization stress. Am J Dent. 2009;22:206–10.
Urban VM, Machado AL, Alves MO, Maciel AP, Vergani CE, Leite ER. Glass transition temperature of hard chairside reline materials after post-polymerisation treatments. Gerodontology. 2010;27:230–5.
De Santis R, Gloria A, Sano H, Amendola E, Prisco D, Mangani F, Rengo S, Ambrosio L, Nicolais L. Effect of light curing and dark reaction phases on the thermomechanical properties of a Bis-GMA based dental restorative material. J Appl Biomater Biomech. 2009;7:132–40.
Ferrante M, Petrini M, Trentini P, Spoto G. Evaluation of composites light-curing at different times and distances of irradiation. J Therm Anal Calorim. 2012;107:757–61.
Khalil SKH, Atkins EDT. Investigation of glass-ionomer cements using differential scanning calorimetry. J Mater Sci Mater Med. 1998;9:529–33.
Berzins DW, Abey S, Costache MC, Wilkie CA, Roberts HW. Resin-modified glass-ionomer setting reaction competition. J Dent Res. 2010;89:82–6.
Micelli F, Maffezolli A, Terzi R, Luprano VA. Characterization of the kinetic behavior of resin modified glass-ionomer cements by DSC, TMA and ultrasonic wave propagation. J Mater Sci Mater Med. 2001;12:151–6.
Nicholson JW, Czarnecka B. Kinetic studies of water uptake and loss in glass-ionomer cements. J Mater Sci Mater Med. 2008;19:1723–7.
Small ICB, Watson TF, Chadwick AV, Sidhu SK. Water sorption in resin-modified glass-ionomer cements: an in vitro comparison with other materials. Biomaterials. 1998;19:545–50.
Akashi A, Matsuya Y, Unemori M, Akamine A. The relationship between water absorption characteristics and the mechanical strength of resin-modified glass-ionomer cements in long-term water storage. Biomaterials. 1999;20:1573–8.
Sidhu SK, Sherriff M, Watson TF. The effects of maturity and dehydration shrinkage on resin-modified glass-ionomer restorations. J Dent Res. 1997;76:1495–501.
Nicholson JW, Anstice HM, McLean JW. A preliminary report on the effect of storage in water on the properties of commercial light-cured glass-ionomer cements. Br Dent J. 1992;173:98–101.
Sidhu SK, Pilecki P, Sherriff M, Watson TF. Crack closure on rehydration of glass ionomer materials. Eur J Oral Sci. 2004;112:465–9.
Brady JE, Senese F. Chemistry: the study of matter and its changes. 5th ed. New York: Wiley; 2007. p. 557–62.
Sorai, M. (ed). Comprehensive handbook of calorimetry and thermal analysis. West Sussex: Wiley; 2004. p. 224–5.
Kanchanavasita W, Anstice HM, Pearson GJ. Water sorption characteristics of resin-modified glass-ionomer cements. Biomaterials. 1997;18:343–9.
McNeill IC, Sadeghi SMT. Thermal stability and degradation mechanisms of poly(acrylic acid) and its salts: Part 1—poly(acrylic acid). Polym Degrad Stab. 1990;29:233–46.
McNeil IC, Sadeghi SMT. Thermal stability and degradation mechanisms of poly(acrylic acid) and its salts: Part 3—magnesium and calcium salts. Polym Degrad Stab. 1990;30:267–82.
Nicholson JW, Wilson AD. Thermal behaviour of films of partially neutralized poly(acrylic acid). 1: influence of metal ions. Br Polym J. 1987;19:67–72.
Ferrante M, Petrini M, Trentini P, Ciavarelli L, Spoto G. Thermal analysis of light curing composites. J Therm Anal Calorim. 2010;102:107–11.
Demirelli K, Coşkun M, Kaya E. A detailed study of thermal degradation of poly(2-hydroxyethyl methacrylate). Polym Degrad Stab. 2001;72:75–80.
Miyazaki CL, Medeiros IS, Matos JR, Filho LER. Thermal characterization of dental composites by TG/DTG and DSC. J Therm Anal Calorim. 2010;102:361–7.
Rojas SS, Frigo GJM, Bernardi MIB, Rastelli ANS, Hernandes AC, Bagnato VS. Thermal and structural properties of commercial dental resins light-cured with blue emitting diodes (LEDs). J Therm Anal Calorim. 2010;99:263–8.
Almeida CC, Mothé CG. Characterization of dental composites by thermal analysis, infrared spectroscopy and scanning electron microscopy. J Therm Anal Calorim. 2009;97:585–9.
Bernardi MIB, Rojas SS, Andreeta MRB, Rastelli ANS, Hernandes AC, Bagnato VS. Thermal analysis and structural investigation of different dental composite resins. J Therm Anal Calorim. 2008;94:791–6.
Acknowledgements
The authors are grateful for the generosity of the manufacturers who supplied the glass-ionomer materials for this study.
Disclaimer
The authors have not financial interest in any of the materials used in this evaluation and any use does not imply endorsement. Any opinions expressed in this work represent those of the authors only and do not represent the official opinions of the United States Air Force, Department of Defense, or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roberts, H., Berzins, D. Thermal analysis of contemporary glass-ionomer restorative materials. J Therm Anal Calorim 115, 2099–2106 (2014). https://doi.org/10.1007/s10973-013-3428-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3428-1