Abstract
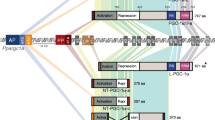
Peroxisome proliferator-activated receptors (PPARs) constitute a subfamily of nuclear receptor superfamily. A wide variety of compounds including hypolipidemic agents, antidiabetic drugs, and long-chain fatty acids are the potential ligands of PPARs. To approach the regulatory mechanisms of PPARs, we studied on two subjects in this work. First, we identified a functional PPAR-binding site in the spacer region between the PEX11α and perilipin genes, which are arranged in tandem on the mouse genome. By gene reporter assays and in vivo as well as in vitro binding assays, we show that these genes are regulated tissue-selectively through this common binding site: The PEX11α gene is activated by PPARα in the liver, whereas the perilipin gene by PPARγ in the adipose tissue. As the second subject, we found that PPARγ2 is conjugated with small ubiquitin-related modifier (SUMO) at a specific lysine residue in the amino-terminal region. By site-directed mutagenesis combined with gene reporter assays and sumoylation analyses, we show that sumoylation represses the ligand-independent transactivating function carried by this region, and hence negatively regulates the whole transactivating competence of PPARγ2. In addition, phosphorylation at a specific site in the amino-terminal region represses the transactivation by PPARγ2 possibly through enhancing sumoylation.
Similar content being viewed by others
References
Desvergne B, Wahli W: Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev 20: 649–688, 1999
Issemann I, Green S: Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650, 1990
Reddy JK, Hashimoto T: Peroxisomal β-oxidation and peroxisome proliferator-activated receptor α: an adaptive metabolic system. Annu Rev Nutr 21: 193–230, 2001
Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM: Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 358: 771–774, 1992
Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S: The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J 11: 433–439, 1992
Palmer CN, Hsu MH, Griffin HJ, Johnson EF: Novel sequence determinants in peroxisome proliferator signaling. J Biol Chem 270: 16114–16121, 1995
Osada S, Tsukamoto T, Takiguchi M, Mori M, Osumi T: Identification of an extended half-site motif required for the function of peroxisome proliferator-activated receptor α. Genes Cells 2: 315–327, 1997
Juge-Aubry C, Pernin A, Favez T, Burger AG, Wahli W, Meier CA, Desvergne B: DNA binding properties of peroxisome proliferator-activated receptor subtypes on various natural peroxisome proliferator response elements. Importance of the 5′-flanking region. J Biol Chem 272: 25252–25259, 1997
Tontonoz P, Hu E, Spiegelman BM: Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79: 1147–1156, 1994
Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ: Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ). Mol Cell Biol 20: 5119–5128, 2000
Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM: Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113: 159–170, 2003
Lemberger T, Desvergne B, Wahli W: Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Biol 12: 335–363, 1996
Berger J, Moller DE: The mechanisms of action of PPARs. Annu Rev Med 53: 409–435, 2002
Lehmann JM, Moore LB, Smith OT, Wilkison WO, Willson TM, Kliewer SA: An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem 270: 12953–12956, 1995
Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM: Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci USA 94: 4318–4323, 1997
Forman BM, Chen J, Evans RM: Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA 94: 4312–4317, 1997
Distel B, Erdmann R, Gould SJ, Blobel G, Crane DI, Cregg JM, Dodt G, Fujiki Y, Goodman JM, Just WW, Kiel JA, Kunau WH, Lazarow PB, Mannaerts GP, Moser HW, Osumi T, Rachubinski RA, Roscher A, Subramani S, Tabak HF, Tsukamoto T, Valle D, van der Klei I, van Veldhoven PP, Veenhuis M: A unified nomenclature for peroxisome biogenesis factors. J Cell Biol 135: 1–3, 1996
Li X, Baumgart E, Dong GX, Morrell JC, Jimenez-Sanchez G, Valle D, Smith KD, Gould SJ: PEX11α is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor α-mediated peroxisome proliferation. Mol Cell Biol 22: 8226–8240, 2002
Abe I, Okumoto K, Tamura S, Fujiki Y: Clofibrate-inducible, 28-kDa peroxisomal integral membrane protein is encoded by PEX11. FEBS Lett 431: 468–472, 1998
Schrader M, Reuber BE, Morrell JC, Jimenez-Sanchez G, Obie C, Stroh TA, Valle D, Schroer TA, Gould SJ: Expression of PEX11β mediates peroxisome proliferation in the absence of extracellular stimuli. J Biol Chem 273: 29607–29614, 1998
Passreiter M, Anton M, Lay D, Frank R, Harter C, Wieland FT, Gorgas K, Just WW: Peroxisome biogenesis: Involvement of ARF and coatomer. J Cell Biol 141: 373–383, 1998
Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK: Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272: 5128–5132, 1997
Werman A, Hollenberg A, Solanes G, Bjørbæ k C, Vidal-Puig AJ, Flier JS: Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor γ (PPARγ). Differential activity of PPARγ1 and -2 isoforms and influence of insulin. J Biol Chem 272: 20230–20235, 1997
Hu E, Kim JB, Sarraf P, Spiegelman BM: Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 274: 2100–2103, 1996
Shimizu M, Takeshita A, Tsukamoto T, Gonzalez FJ, Osumi T: Tissue-selective, bidirectional regulation of PEX11α and perilipin genes through a common peroxisome proliferator response element. Mol Cell Biol 24: 1313–1323, 2004
Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T: The transactivating function of peroxisome proliferator-activated receptor γ is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells 9: 1017–1029, 2004
Hi R, Osada S, Yumoto N, Osumi T: Characterization of the amino-terminal activation domain of peroxisome proliferator-activated receptor α. Importance of α-helical structure in the transactivating function. J Biol Chem 274: 35152–35158, 1999
Sambrook J, Fritsch, EF, Maniatis T: Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: cold Spring Harbor Laboratory Press. p. 16.32–16.36, 1989
Tominaga S, Morikawa M, Osumi T: Growth hormone has dual stage-specific effects on the differentiation of 3T3-L1 preadipocytes. J Biochem (Tokyo) 132: 881–889, 2002
Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C: Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266: 11341–11346, 1991
Servetnick DA, Brasaemle DL, Gruia-Gray J, Kimmel AR, Wolff J, Londos C: Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and leydig cells. J Biol Chem 270: 16970–16973, 1995
Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ: Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem 275: 5011–5015, 2000
Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C: Perilipin a is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161: 1093–1103, 2003
Seeler JS, Dejean A: Nuclear and unclear functions of sumo. Nat Rev Mol Cell Biol 4: 690–699, 2003
Gill G: Post-translational modification by the small ubiquitin-related modifier sumo has big effects on transcription factor activity. Curr Opin Genet Dev 13: 108–113, 2003
Verger A, Perdomo J, Crossley M: Modification with SUMO. A role in transcriptional regulation. EMBO Rep 4: 137–142, 2003
Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR: Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med 339: 953–959, 1998
Adachi N, Lieber MR: Bidirectional gene organization: a common architectural feature of the human genome. Cell 109: 807–809, 2002
Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R: The peroxisome proliferator-activated receptor γ regulates expression of the perilipin gene in adipocytes. J Biol Chem 279: 10070–10076, 2004
Dalen KT, Schoonjans K, Ulven SM, Weedon-Fekjaer MS, Bentzen TG, Koutnikova H, Auwerx J, Nebb HI: Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-γ. Diabetes 53: 1243–1252, 2004
Nagai S, Shimizu C, Umetsu M, Taniguchi S, Endo M, Miyoshi H, Yoshioka N, Kubo M, Koike T: Identification of a functional peroxisome proliferator-activated receptor responsive element within the murine perilipin gene. Endocrinology 145: 2346–2356, 2004
Ohshima T, Koga H, Shimotohno K: Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem 279: 29551–29557, 2004
Floyd ZE, Stephens JM: Control of peroxisome proliferator-activated receptor γ2 stability and activity by sumoylation. Obes Res 12: 921–928, 2004
Osumi T, Wen JK, Hashimoto T: Two cis-acting regulatory sequences in the peroxisome proliferator-responsive enhancer region of rat acyl-CoA oxidase gene. Biochem Biophys Res Commun 175: 866–871, 1991
Author information
Authors and Affiliations
Corresponding author
Additional information
MS and DY equally contributed to this work.
Rights and permissions
About this article
Cite this article
Shimizu, M., Yamashita, D., Yamaguchi, T. et al. Aspects of the regulatory mechanisms of PPAR functions: Analysis of a bidirectional response element and regulation by sumoylation. Mol Cell Biochem 286, 33–42 (2006). https://doi.org/10.1007/s11010-005-9052-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-9052-z