Abstract
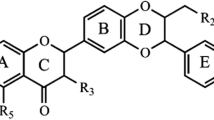
Silymarin is a naturally available bioflavonoid and is a strong antioxidant with a capacity to inhibit the formation of tumors in several cancer models. In the present study, we investigated whether dietary supplementation of silymarin has any role in lipid components, lipid-metabolizing enzymes, free fatty acid profile, and expression of cyclooxygenase-2 (COX-2) in N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinoma in rats. NDEA-induced rats showed severe hyperlipidemia along with upregulated expression of COX-2 as revealed by western blotting and immunohistochemistry. Dietary silymarin supplementation attenuated this hyperlipidemia and downregulated the expression of COX-2. Thus we conclude that compounds like silymarin with potent hypolipidemic effect are strong candidates as chemopreventive agents for the treatment of liver cancer.






Similar content being viewed by others
References
Jemal A, Murray T, Ward E et al (2005) Cancer statistics. CA Cancer J Clin 55:10–30
Leong TYM, Leong ASY (2005) Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB 7:5–15
Chang CK, Astrakianakis G, Thomas DB et al (2006) Occupational exposures and risks of liver cancer among Shanghai female textile workers—a case-cohort study. Int J Epidemiol 35:361–369
Yamamoto J, Kosuge T, Takayama T et al (1996) Recurrence of hepatocellular carcinoma after surgery. Br J Surg 83:1219–1222
Ramakrishnan G, Raghavendran HRB, Vinodhkumar R, Devaki T (2006) Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact 161:104–114
Mittal G, Brar APS, Soni G (2006) Impact of hypercholestrolemia on toxicity of N-nitrosodiethylamine: biochemical and histopathological effects. Pharmacol Rep 58(3):413–419
Liao DJ, Blanck A, Eneroth P, Gustafsson JA, Hällström IP (2001) Diethylnitrosamine causes pituitary damage, disturbs hormone levels and reduces sexual dimorphism of certain liver functions in the rat. Environ Health Perspect 109:943–947
IARC (1971) Monograph on the evaluation of carcinogenic risk of chemicals to man, vol 1. International Agency for Research on Cancer, Lyon, pp 107–124
Yanaida Y, Kohno H, Yoshida K et al (2002) Dietary silymarin suppresses 4-nitroquinoline 1-oxide induced tongue carcinogenesis in male F344 rats. Carcinogenesis 23(5):787–794
Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S (2007) Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 560:110–116
Jiang J, Ehle PN, Xu N (2006) Influence of liver cancer on lipid and lipoprotein metabolism (review). Lipids Health Dis 5:4
Tang TC, Poon RT, Lau CP, Xie D, Fan ST (2005) Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol 11(13):1896–1902
Ramakrishnan G, Augustine TA, Jagan S, Vinodhkumar R, Devaki T (2007) Effect of silymarin on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Exp Oncol 29(1):39–44
Folch J, Lees M, Stanley GHS (1957) A simple methods for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Parekh AC, Jung DH (1970) Cholesterol determination with ferric aceta-uranyl acetate and sulphuric acid-ferrous sulphate reagents. Anal Chem 42:1423–1427
Foster LB, Dunn RT (1973) Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin Chem 19:338–340
Hron WT, Menahan LA (1981) A sensitive method for the determination of free fatty acids in plasma. J Lipid Res 122:377–381
Bartlett GR (1959) Phosphorus assay by column chromatography. J Biol Chem 234:466–468
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Burstein M, Scholnick HR (1972) Precipitation of chylomicron and very low density lipoprotein from human serum with sodium lauryl sulphate. Life Sci 11:177–184
Bier M (1955) Enzymes of lipid metabolism. Lipases and esterases. Methods Enzymol 1:631–638
Hitz J, Sternmetz J, Siest G (1983) Plasma LCAT-reference values and effects of xenobiotics. Clin Chim Acta 133:85–96
Schmidt A (1974) Measurement of lipoprotein lipase and hepatic triglyceride lipase in human post heparin plasma. Methods Enzymol 72:325–337
Rao AV, Ramakrishnan S (1972) Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem 21(10):1523–1525
Morrison WR, Smith LM (1964) Preparations of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride methanol. J Lipid Res 5:600–607
Sako A, Kitayama J, Kaisaki S, Nagawa H (2004) Hyperlipidemia is a risk factor for lymphatic metastasis in superficial esophageal carcinoma. Cancer Lett 208:43–49
Kitayama J, Hatano K, Kaisaki S, Suzuki H, Fujii S, Nagawa H (2004) Hyperlipidemia is positively is correlated with lymphatic metastasis in men with early gastric cancers. Br J Surg 91(2):191–198
Takahashi HK, Schmidt GW, Iwagaki H, Yoshino T, Tanaka N, Nishibori M (2006) Hypothesis: the antitumor activities of statins may be mediated by IL-18. J Leukoc Biol 80:215–216
Kawasaki M, Yagasaki K, Miura Y, Funabiki R (2004) Comparison of the changes in lipid metabolism between hepatoma-bearing and lipopolysaccharide-treated rats. Biosci Biotechnol Biochem 68(1):72–78
Hirayama T, Honda A, Matsuzaki Y et al (2006) Hypercholesterolemia in rats with hepatomas: increased oxysterols accelerate efflux but do not inhibit biosynthesis of cholesterol. Hepatology 44(3):602–611
Regnstrom J, Nisson J, Toruvall P, Laudou C, Hamsten A (1992) Susceptibility to low-density lipoproteins oxidation and coronary atherosclerosis in man. Lancet 339:1883–1886
Chander R, Kapoor NK (1990) High density lipoprotein is a scavenger of superoxide anions. Biochem Pharmacol 40:1663–1665
Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM (2005) Statins and cancer prevention. Nat Rev Cancer 5(12):930–942
Asakage M, Tsuno NH, Kitayam J (2004) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (pravastatin) inhibits endothelial cell proliferation dependent on G1 cell cycle arrest. Anticancer Drugs 15(6):625–632
Dehmlow C, Murawski N, de Groot H et al (1996) Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci 58:1591–1600
Kolanjiappan K, Ramachandran CR, Manoharan S (2003) Biochemical changes in tumor tissues of oral cancer patients. Clin Biochem 36:61–65
Carbo N, Costelli P, Tessitore L et al (1994) Anti-tumour necrosis factor treatment interferes with changes in lipid metabolism in a tumour cachexia model. Clin Sci 87:349–355
Mills GB, Moolenaar WH (2003) The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 8:582–591
Thirunavukkarasu C, Selvendhiran K, Princevijeyasingh J, Senthilnathan P, Sakthisekaran D (2003) Effect of sodium selenite on lipids and lipid-metabolizing enzymes in N-nitrosodiethylamine-induced hepatoma-bearing rats. J Trace Elem Exp Med 16(1):1–15
Rogers MP, Hutchinson I (1981) The effect of in vitro high-density lipoprotein on hydrolysis of triacyl glycerol by lipoprotein lipase. Biochem J 200:453–457
Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G (2007) Arachidonic acid and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact 165:239–250
Sravankumar G, Das UN (1997) Cytotoxic action of alpha linolenic and eicosapentaenoic acids on myeloma cells in vitro. Prostaglandins Leukot Essent Fatty Acids 56:285–293
Vento R, Alessandro ND, Giuliano M, Lauricells M, Carabillo M, Tesoriere G (2000) Induction of apoptosis by arachidonic acid in human retinoblastoma Y79 cells: involvement of oxidative stress. Exp Eye Res 70(4):503–517
Bianchi A, Dewailly E, Gautier H et al (2004) Decrease of human hepatoma cell growth by arachidonic acid is associated with an accumulation of derived products from lipid peroxidation. Biochimie 86:633–642
Jones R, Alvarez LFA, Alvarez OR, Broaddus R, Das S (2006) Arachidonic acid and colorectal carcinogenesis. Mol cell Biochem 253:141–149
Koga H (2003) Hepatocellular carcinoma: is there a potential for chemoprevention using cyclooxygenase-2 inhibitors? Cancer 98:661–667
Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T (2003) Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through AKT activation: evidence for AKT inhibition in celecoxib-induced apoptosis. Hepatology 38:756–768
Acknowledgments
The authors wish to thank Dr. CEAAL Analytical Laboratory (A unit of C. L. Baid Mehta College of Pharmacy), Chennai, for their help in Gas chromatography analysis and we wish to thank Dr. P. Srinivasan, Korea Atomic Energy Research Institute, South Korea, for his guidance and assistance during immunohistochemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramakrishnan, G., Elinos-Báez, C.M., Jagan, S. et al. Silymarin downregulates COX-2 expression and attenuates hyperlipidemia during NDEA-induced rat hepatocellular carcinoma. Mol Cell Biochem 313, 53–61 (2008). https://doi.org/10.1007/s11010-008-9741-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9741-5