Abstract
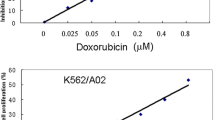
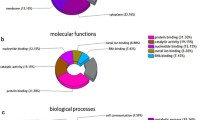
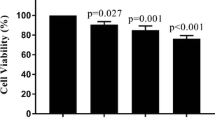
This study aimed to explore the mechanism of adriamycin resistance in human chronic myelogenous leukemia cells. Proteomic approach was utilized to compare and identify differentially expressed proteins between human chronic myelogenous leukemia K562 cells and their adriamycin-resistant counterparts. The differentially expressed proteins were analyzed by 2-DE (two-dimensional gel electrophoresis), and protein identification were performed on ESI-Q-TOF MS/MS instrument. Out of the 35 differentially expressed proteins between the two cell lines, 29 were identified and grouped into 10 functional classes. Most of identified proteins were related to the categories of metabolism (24%), proteolysis (13%), signal transduction (21%) and calcium ion binding (6%), suggesting that alterations of those biological processes might be involved in adriamycin resistance of K562 cells. We believe this study may provide some clues to a better understanding of the molecular mechanisms underlying adriamycin resistance.





Similar content being viewed by others
Abbreviations
- 2-DE:
-
Two-dimensional gel electrophoresis
- ACN:
-
Acetonitrile
- CBB:
-
Coomassie brilliant blue
- DMEM:
-
Dulbecco-modified Eagle medium
- IEF:
-
Isoelectric focusing
- IPG:
-
Immobilized pH gradient
- MS:
-
Mass spectrometry
- PBS:
-
Phosphate-buffered saline
- PVDF:
-
Polyvinylidene fluoride
- Q-TOF:
-
Quadrupole time-of-flight
- TBST:
-
Tris-buffered saline Tween-20
References
Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Faneyte IF, Kristel PM, Maliepaard M et al (2002) Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res 8:1068–1074
Walker J, Martin C, Callaghan R (2004) Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. Eur J Cancer 40:594–605
Ruiz de Almodovar C, Ruiz-Ruiz C, Munoz-Pinedo C et al (2001) The differential sensitivity of Bcl-2-overexpressing human breast tumor cells to TRAIL or doxorubicin-induced apoptosis is dependent on Bcl-2 protein levels. Oncogene 20:7128–7133
Gariboldi MB, Ravizza R, Riganti L et al (2003) Molecular determinants of intrinsic resistance to doxorubicin in human cancer cell lines. Int J Oncol 22:1057–1064
Pommier Y, Sordet O, Antony S et al (2004) Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 23:2934–2949
Prost S (1995) Mechanisms of resistance to topoisomerases poisons. Gen Pharmacol 26:1673–1684
Vimalachandran D, Costello E (2004) Proteomic technologies and their application to pancreatic cancer. Expert Rev Proteomics 1:493–501
Chen R, Pan S, Brentnall TA, Aebersold R (2005) Proteomic profiling of pancreatic cancer for biomarker discovery. Mol Cell Proteomics 4:523–533
Yang YX, Sun XF, Cheng AL et al (2009) Increased expression of HSP27 linked to vincristine resistance in human gastric cancer cell line. J Cancer Res Clin Oncol 135:181–189
Shi YQ, Hu WH, Yin F et al (2004) Regulation of drug sensitivity of gastric cancer cells by human calcyclin-binding protein (CacyBP). Gastric Cancer 7:160–166
Chen R, Yi EC, Donohoe S et al (2005) Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology 129:1187–1197
Shekouh AR, Thompson CC, Prime W et al (2003) Application of laser capture microdissection combined with two-dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics 3:1988–2001
Zhu F, Wang Y, Zeng S, Fu X, Wang L, Cao J (2009) Involvement of Annexin A1 in multidrug resistance of K562/ADR cells identified by the proteomic study. OMICS 13:467–476
Tong A, Zhang H, Li Z et al (2007) Proteomic analysis of liver cancer cells treated with suberonylanilide hydroxamic acid. Cancer Chemother Pharmacol 61:791–802
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276
Brown JM (1999) The hypoxic cell: a target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 59:5863–5870
Evans SM, Koch CJ (2003) Prognostic significance of tumor oxygenation in humans. Cancer Lett 195:1–16
Bos R, Groep PVD, Greijer AE et al (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97:1573–1581
Teicher BA (1994) Hypoxia and drug resistance. Cancer Metastasis Rev 13:139–168
Voorhees PM, Dees EC, O’Neil B, Orlowski RZ (2003) The proteasome as a target for cancer therapy. Clin Cancer Res 9:6316–6325
Ruggeri B, Miknyoczki S, Dorsey B et al (2009) The development and pharmacology of proteasome inhibitors for the management and treatment of cancer. Adv Pharmacol 57:91–135
Milano A, Iaffaioli RV, Caponigro F (2007) The proteasome: a worthwhile target for the treatment of solid tumours? Eur J Cancer 43:1125–1133
Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4:349–360
Maniatis T (1999) A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev 13:505–510
Ma MH, Yang HH, Parker K et al (2003) The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res 9:1136–1144
Olofsson B (1999) Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal 11:545–554
Sasaki T, Takai Y (1998) The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun 245:641–645
Golovanov AP, Chuang TH, DerMardirossian C et al (2001) Structure–activity relationships in flexible protein domains: regulation of rho GTPases by RhoGDI and D4 GDI. J Mol Biol 305:121–135
Fukumoto Y, Kaibuchi K, Hori Y et al (1990) Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 5:1321–1328
Poland J, Schadendorf D, Lage H et al (2002) Study of therapy resistance in cancer cells with functional proteome analysis. Clin Chem Lab Med 40:221–234
Sinha P, Kohl S, Fischer J et al (2000) Identification of novel proteins associated with the development of chemoresistance in malignant melanoma using two-dimensional electrophoresis. Electrophoresis 21:3048–3057
Takano M, Goto T, Sakamoto M et al (2003) Identification of paclitaxel-resistance related genes by differential display using cDNA microarray in ovarian cancer cell lines. Proc Am Soc Clin Oncol 22:462
Zhang BL, Zhang YQ, Dagher MC et al (2005) Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res 65:6054–6062
Zhang F, Zhang L, Zhang B et al (2009) Anxa2 plays a critical role in enhanced invasiveness of multidrug resistant human breast cancer cells. J Proteome Res 8:5041–5047
Kim A, Enomoto T, Serada S et al (2009) Enhanced expression of Annexin A4 in clear cell carcinoma of the ovary and its association with chemoresistance to carboplatin. Int J Cancer 125:2316–2322
Hütter G, Sinha P (2001) Proteomics for studying cancer cells and the development of chemoresistance. Proteomics 1:1233–1248
Sinha P, Poland J, Kohl S et al (2003) Study of the development of chemoresistance in melanoma cell lines using proteome analysis. Electrophoresis 24:2386–2404
Murphy L, Henry M, Meleady P, Clynes M, Keenan J (2008) Proteomic investigation of taxol and taxotere resistance and invasiveness in a squamous lung carcinoma cell line. Biochim Biophys Acta 1784:1184–1191
Acknowledgments
This study was supported by the grants from the National Natural Sciences Foundation of China (30801294), Fok Ying Tung Education Foundation (201080) and the special major science and technology project for “creation of major new drugs” of China (2009ZX09103-132).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xingchen Peng and Fengming Gong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Peng, X., Gong, F., Xie, G. et al. A proteomic investigation into adriamycin chemo-resistance of human leukemia K562 cells. Mol Cell Biochem 351, 233–241 (2011). https://doi.org/10.1007/s11010-011-0730-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0730-8