Abstract
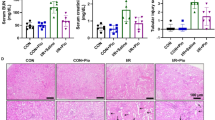
Endothelial nitric-oxide synthase (eNOS) acts as a common pathogenic pathway in diabetic nephropathy (DN). However, its functional consequences are still not fully understood. Caveolin, a membrane protein, inhibits the eNOS by making caveolin–eNOS complex, and its expression is upregulated during diabetes mellitus (DM). This study was designed to determine the role of caveolin in eNOS-mediated NO synthesis and release in DN. DM in rat was induced by feeding of high-fat diet (HFD) for 2 weeks, followed by single dose of streptozotocin (STZ) (35 mg/kg, ip) further followed by HFD for further 8 weeks. Serum nitrite/nitrate ratio was measured to determine the plasma level of NO. Diabetic rat, after 6 weeks of STZ, developed elevated level of BUN, protein in urine, urinary output, serum creatinine, serum cholesterol, kidney weight, kidney weight/body weight, and renal cortical collagen content, while serum nitrite/nitrate concentration was significantly decreased as compared to normal control group. Treatment with sodium nitrite (NO donor), l-arginine (NO precursor), daidzein (caveolin inhibitor), and combination of l-arginine and daidzein for 2 weeks markedly attenuated these changes and increased serum nitrite/nitrate ratio. However, treatment with L-NAME, a eNOS inhibitor, significantly attenuated the l-arginine-, daidzein-, or combination of l-arginine and daidzein-induced ameliorative effects in DN. The finding of this study suggests that caveolin plays a vital role in the eNOS-mediated decrease in renal level of NO, which may be responsible for the development of DN in rats.











Similar content being viewed by others
References
Ichinose K, Kawasaki E, Eguchi K (2007) Recent advancements of understanding pathogenesis of Type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol 27:554–564
Marshall SM (2004) Recent advances in diabetic nephropathy. Clin Med 3:277–282
Prabhakar S, Starnes J, Shi S, Lonis B, Tran R (2007) Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol 18:2945–2952
Vaishya R, Singh J, Lal H (2008) Effect of irbesartan on streptozotocin-induced diabetic nephropathy: an interventionary study. Indian J Clin Biochem 23:195–197
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR (2008) Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med 233:4–11
Onozato ML, Tojo A (2005) Role of NADPH oxidase in hypertension and diabetic nephropathy. Curr Hypertens Rev 1:15–20
Tomlinson DR (1999) Mitogen-activated protein kinases as glucose transducers for diabetic complications. Diabetologia 42:1271–1281
Navarro-Gonzalez JF, Mora-Fernandez C (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19:433–442
Dronavalli S, Duka I, Babris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4:444–452
Brewster UC, Setaro JF, Perazella MA (2003) The renin angiotensin aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci 326:15–24
Anderson S (1994) Relevance of single nephron studies to human glomerular function. Kidney Int 45:384–389
Wiecek A, Chudek J, Kokot F (2003) Role of angiotensin II in progression of diabetic nephropathy-therapeutic implications. Nephrol Dial Transpl 8:v16–v20
Hilger KF, Veelken R (2005) Type 2 diabetic nephropathy: never too early to treat? J Am Soc Nephrol 16:574–575
Sedding DG, Braun-Dullaeus RC (2006) Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med 1:50–55
Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Christ GJ, Edelmann W, Lisanti MP (2002) Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 22:2329–2344
Hardin CD, Vallejo J (2006) Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res 69:808–815
Frank PG, Woodman SE, Park DS, Lisanti MP (2003) Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 23:1161–1168
Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC (2000) In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6:1362–1367
Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL (1999) Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest 103:897–905
Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene disrupted mice. Science 293:2449–2452
Escandon JC, Cipolla M (2001) Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev 22:36–52
Ulker S, McKeown P, Bayraktutan U (2003) Vitamins reverse endothelial dysfunction through regulation of eNOS and NADPH oxidase activities. Hypertension 41:534–541
Lott JA, Turner K (1975) Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem 21:1754–1760
Jamall IS, Finelli VN, Que Hee SS (1981) A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem 112:70–75
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Srinivasan K, Viswanad B, Lydia Asrat, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed, low-dose streptozotocin-treated rat. A model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320
Shah DI, Singh M (2006) Inhibition of protein tyrosin phosphatase improves vascular endothelial dysfunction. Vasc Pharmacol 44:177–182
Jindal S, Singh M, Balakumar P (2007) Effect of bis (maltolato) oxovanadium (BMOV) in uric acid and sodium arsenite-induced vascular endothelial dysfunction in rats. Int J Cardiol 128:383–391
Zhao HJ, Wang S, Cheng H, Zhang Z, Takahashi T, Fogo AB, Breyer MD, Harris RC (2006) Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17:2664–2669
Fujisawa G, Okada K, Muto S, Futija N, Itabashi N, Kusano E, Ishibashi S (2004) Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney int 66(4):1493–1502
Sinuani I, Averbukh Z, Gitelman I, Rapoport MJ, Sandbank J, Albeck M, Sredni B, Weissgarten J (2006) Mesangial cells initiate compensatory renal tubular hypertrophy via IL-10-induced TGF-beta secretion: effect of the immunomodulator AS101 on this process. Am J Physiol Renal Physiol 291:F384–F394
Ahmad J (2008) Renin angiotensin system blockade in diabetic nephropathy. Diabetes and metabolic syndrome. Clin Res Rev 2:135–158
Patzak A, Lai E, Persson PB, Persson AE (2005) Angiotensin II-nitric oxide interaction in glomerular arterioles. Clin Exp Pharmacol Physiol 32:410–414
Schricker K, Kurtz A (1993) Liberators of NO exert a dual effect on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol 265:F180–F186
Xiao-Rui He, Suzanne G, Greenberg Josie P, Jurgen B (1995) Effect of nitric oxide in renin secretion II. Studies in the perfused juxtaglomerular apparatus. Am J Physiol 268:F953–F959
Greenberg SG, He XR, Schnermann JB, Briggs JP (1995) Effect of nitric oxide on renin secretion I. Studies in isolated juxtaglomerular granular cells. Am J Physiol 268:F948–F952
Laursen JB, Boesgaard S, Trautner S, Rubin I, Poulsen HE, Aldershvile J (2001) Endothelium-dependent vasorelaxation is inhibited by in vivo depletion of vascular thiol levels: role of endothelial nitric oxide synthase. Free Radic Res 35:387–394
Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS (2002) Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int 61:186–194
Nakagawa T, Sato W, Glushakova W, Heinig M, Clarke T, Thompson M (2007) Diabetic endothelial nitric oxide synthase knock out mice develops advanced diabetic nephropathy. J Am Soc Nephrol 18:539–550
Chini B, Parenti M (2004) G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol 32:325–338
Sessa WC (2005) Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz 100:15–18
Woodman OL, Missen MA, Boujaoude M (2004) Daidzein and 17 betaestradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol 44:155–163
Peterson TE, Kleppe LS, Caplice NM, Pan S, Mueske CS, Simari RD (1999) The regulation of caveolin expression and localization by serum and heparin in vascular smooth muscle cells. Communication 265:722–727
Acknowledgments
We are grateful to Mr. Praveen Garg, Chairman ISF College of Pharmacy, Moga for his support and encouragement during the conduct of this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arya, A., Yadav, H.N. & Sharma, P.L. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Mol Cell Biochem 354, 57–66 (2011). https://doi.org/10.1007/s11010-011-0805-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0805-6