Abstract
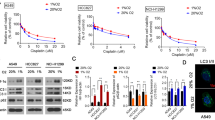
A disintegrin and metalloproteinase-17 (ADAM17) is a member of the metalloproteinase superfamily and involved in the cleavage of ectodomain of many transmembrane proteins. ADAM17 is overexpressed in a variety of human tumors, which is associated with tumor development and progression. In the present study, we sought to investigate the expression and function of ADAM17 in hypoxia-treated hepatocellular carcinoma (HCC) cells. Western blot analysis was used to measure the expression of ADAM17 in HCC cell lines (Hep3B and HepG2 cells). Annexin V/PI double staining was performed to analyze the effects of ADAM17 on hypoxia-mediated cisplatin resistance. ADAM17 expression was upregulated by hypoxia treatment in HCC cells at both mRNA and protein levels. Overexpression of ADAM17 reduced cisplatin-induced apoptosis in HCC cells, accompanies by less cleavage of caspase-3 and poly (ADP-ribose) polymerase (PARP). Forced expression of ADAM17 enhanced the phosphorylation of epidermal growth factor receptor (EGFR) and Akt without affecting the expression of total EGFR and Akt. Pretreatment with EGFR inhibitor AG1478 or phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 rescued ADAM17-mediated cisplatin resistance of HCC cells. ADAM17 silencing attenuated hypoxia-induced cisplatin resistance and enhanced the accumulation of cleaved caspase-3 and PARP. Western blot analysis showed that overexpression of hypoxia-inducible factor-1α (HIF-1α), a transcription factor, upregulated the expression of ADAM17 and HIF-1α silencing downregulated the expression of ADAM17 in hypoxia-treated HCC cells, indicating the regulation of ADAM17 by HIF-1α. Taken together, our results indicated that ADAM17 is upregulated by hypoxia and contributes to hypoxia-induced cisplatin resistance via EGFR/PI3K/Akt pathway.





Similar content being viewed by others
References
Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M (2003) Tumor hypoxia: a target for selective cancer therapy. Cancer Sci 94:1021–1028
Wu XZ, Xie GR, Chen D (2007) Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol 22:1178–1182
Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62:3387–3394
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D (2008) Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 99:121–128
Arsham AM, Plas DR, Thompson CB, Simon MC (2004) Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res 64:3500–3507
Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC (2004) Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res 64:1765–1772
Liu G, Roy J, Johnson EA (2006) Identification and function of hypoxia-response genes in Drosophila melanogaster. Physiol Genomics 25:134–141
Black RA (2002) Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol 34:1–5
Yoshimura T, Tomita T, Dixon MF, Axon AT, Robinson PA, Crabtree JE (2002) ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in Helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J Infect Dis 185:332–340
Ding X, Yang LY, Huang GW, Wang W, Lu WQ (2004) ADAM17 mRNA expression and pathological features of hepatocellular carcinoma. World J Gastroenterol 10:2735–2739
Blanchot-Jossic F, Jarry A, Masson D, Bach-Ngohou K, Paineau J, Denis MG, Laboisse CL, Mosnier JF (2005) Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells. J Pathol 207:156–163
Zheng X, Jiang F, Katakowski M, Zhang ZG, Lu QE, Chopp M (2009) ADAM17 promotes breast cancer cell malignant phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther 8:1045–1054
Szalad A, Katakowski M, Zheng X, Jiang F, Chopp M (2009) Transcription factor Sp1 induces ADAM17 and contributes to tumor cell invasiveness under hypoxia. J Exp Clin Cancer Res 28:129
Rzymski T, Petry A, Kračun D, Rieß F, Pike L, Harris AL, Görlach A (2012) The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene 31:3621–3634
Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, Zhang ZG, Yang H, Chopp M (2007) Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci 98:674–684
Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren ZG, Sun HC, Tang ZY (2010) Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res 16:2740–2750
Etzerodt A, Maniecki MB, Møller K, Møller HJ, Moestrup SK (2010) Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol 88:1201–1205
Lau CK, Yang ZF, Lam SP, Lam CT, Ngai P, Tam KH, Poon RT, Fan ST (2007) Inhibition of Stat3 activity by YC-1 enhances chemo-sensitivity in hepatocellular carcinoma. Cancer Biol Ther 6:1900–1907
Katakowski M, Jiang F, Zheng X, Gutierrez JA, Szalad A, Chopp M (2009) Tumorigenicity of cortical astrocyte cell line induced by the protease ADAM17. Cancer Sci 100:1597–1604
Katakowski M, Chen J, Zhang ZG, Santra M, Wang Y, Chopp M (2007) Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab 27:669–678
Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C (2003) Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem 278:26202–26207
Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J (2003) TACE is required for the activation of the EGFR by TGF-alpha in tumors. EMBO J 22:1114–1124
Kyula JN, Van Schaeybroeck S, Doherty J, Fenning CS, Longley DB, Johnston PG (2010) Chemotherapy-induced activation of ADAM-17: a novel mechanism of drug resistance in colorectal cancer. Clin Cancer Res 16:3378–3389
Kagawa K, Nakano A, Miki H, Oda A, Amou H, Takeuchi K, Nakamura S, Harada T, Fujii S, Yata K, Ozaki S, Matsumoto T, Abe M (2012) Inhibition of TACE activity enhances the susceptibility of myeloma cells to TRAIL. PLoS ONE 7:e31594
Liu WH, Chang LS (2010) Suppression of ADAM17-mediated Lyn/Akt pathways induces apoptosis of human leukemia U937 cells: Bungarus multicinctus protease inhibitor-like protein-1 uncovers the cytotoxic mechanism. J Biol Chem 285:30506–30515
Hilliard VC, Frey MR, Dempsey PJ, Peek RM Jr, Polk DB (2011) TNF-α converting enzyme-mediated ErbB4 transactivation by TNF promotes colonic epithelial cell survival. Am J Physiol Gastrointest Liver Physiol 301:G338–G346
Lizama C, Rojas-Benítez D, Antonelli M, Ludwig A, Bustamante-Marín X, Brouwer-Visser J, Moreno RD (2010) TACE/ADAM17 is involved in germ cell apoptosis during rat spermatogenesis. Reproduction 140:305–317
Arribas J, Esselens C (2009) ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des 15:2319–2335
Zheng X, Jiang F, Katakowski M, Lu Y, Chopp M (2012) ADAM17 promotes glioma cell malignant phenotype. Mol Carcinog 51:150–164
Zheng X, Jiang F, Katakowski M, Zhang X, Jiang H, Zhang ZG, Chopp M (2008) Sensitization of cerebral tissue in nude mice with photodynamic therapy induces ADAM17/TACE and promotes glioma cell invasion. Cancer Lett 265:177–187
Lin P, Sun X, Feng T, Zou H, Jiang Y, Liu Z, Zhao D, Yu X (2012) ADAM17 regulates prostate cancer cell proliferation through mediating cell cycle progression by EGFR/PI3K/AKT pathway. Mol Cell Biochem 359:235–243
Thabet MM, Huizinga TW (2006) Drug evaluation: apratastat, a novel TACE/MMP inhibitor for rheumatoid arthritis. Curr Opin Investig Drugs 7:1014–1019
Sparano JA, Bernardo P, Stephenson P, Gradishar WJ, Ingle JN, Zucker S, Davidson NE (2004) Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J Clin Oncol 22:4683–4690
Tape CJ, Willems SH, Dombernowsky SL, Stanley PL, Fogarasi M, Ouwehand W, McCafferty J, Murphy G (2011) Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci USA 108:5578–5583
Richards FM, Tape CJ, Jodrell DI, Murphy G (2012) Anti-tumour effects of a specific anti-ADAM17 antibody in an ovarian cancer model in vivo. PLoS ONE 7:e40597
Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM (2002) MRI of the tumor microenvironment. J Magn Reson Imaging 16:430–450
Jiang J, Tang YL, Liang XH (2011) EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther 11:714–723
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–1480
Charbonneau M, Harper K, Grondin F, Pelmus M, McDonald PP, Dubois CM (2007) Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor alpha-induced increases in tumor necrosis factor-alpha converting enzyme/ADAM17 expression by synovial cells. J Biol Chem 282:33714–33724
Laemmle A, Lechleiter A, Roh V, Schwarz C, Portmann S, Furer C, Keogh A, Tschan MP, Candinas D, Vorburger SA, Stroka D (2012) Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions. PLoS ONE 7:e33433
Saponaro C, Malfettone A, Ranieri G, Danza K, Simone G, Paradiso A, Mangia A (2013) VEGF, HIF-1α expression and MVD as an angiogenic network in familial breast cancer. PLoS ONE 8:e53070
Zhdanov AV, Dmitriev RI, Papkovsky DB (2012) Bafilomycin A1 activates HIF-dependent signalling in human colon cancer cells via mitochondrial uncoupling. Biosci Rep 32:587–595
Kanda K, Komekado H, Sawabu T, Ishizu S, Nakanishi Y, Nakatsuji M, Akitake-Kawano R, Ohno M, Hiraoka Y, Kawada M, Kawada K, Sakai Y, Matsumoto K, Kunichika M, Kimura T, Seno H, Nishi E, Chiba T (2012) Nardilysin and ADAM proteases promote gastric cancer cell growth by activating intrinsic cytokine signalling via enhanced ectodomain shedding of TNF-α. EMBO Mol Med 4:396–411
Wang YY, Lo GH, Lai KH, Cheng JS, Lin CK, Hsu PI (2003) Increased serum concentrations of tumor necrosis factor-alpha are associated with disease progression and malnutrition in hepatocellular carcinoma. J Chin Med Assoc 66:593–598
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Xj., Feng, Cw. & Li, M. ADAM17 mediates hypoxia-induced drug resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem 380, 57–66 (2013). https://doi.org/10.1007/s11010-013-1657-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1657-z