Abstract
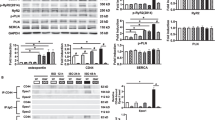
Group I metabotropic glutamate receptors, mGluR1 and mGluR5, are associated with sympathetic nerve activity. Sympathetic nerve stimulation exerts a crucial effect on modulating phosphorylation status and distribution of connexin43 (Cx43) in rat heart. Hence, mGluR1 and mGluR5 have an indirect effect on regulating the function of gap junction channels, which is affected by the availability of Cx43 protein. Additionally, it has been demonstrated that mGluR1/5 are present in ventricular myocardium in particular intercalated disks where Cx43 is the principal component of ventricular gap junction channels. We, therefore, hypothesized that mGluR1/5 might regulate Cx43 phosphorylation and gap junctional intercellular communication (GJIC) directly, independent of sympathetic nerve stimulation. After documenting the presence of mGluR1 and mGluR5 in H9c2 cardiomyoblast cells, addition of the selective mGluR1/5 agonist (S)-3,5-dihydroxyphenylglycine hydrate (DHPG) induced Cx43 phosphorylation and GJIC inhibition in both concentration- and time-dependent manner. The effects of DHPG were abolished by the mGluR1 antagonist LY367385 and the specific inhibitor of MEK1, PD98059 which also reduced phosphorylation of extracellular-signal-regulated protein kinase 1/2 (ERK1/2); but not by the mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine hydrochloride or the selective inhibitor of protein kinase C (PKC). In conclusion, in H9c2 cardiomyoblast cells mGluR1 increases Cx43 phosphorylation level and suppresses GJIC involving ERK1/2 but not PKC.





Similar content being viewed by others
References
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. doi:10.1146/annurev.pharmtox.37.1.205
Dalfo E, Albasanz JL, Rodriguez A, Martin M, Ferrer I (2005) Abnormal group I metabotropic glutamate receptor expression and signaling in the frontal cortex in Pick disease. J Neuropathol Exp Neurol 64:638–647
Conn PJ (2003) Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci 1003:12–21
Peavy RD, Chang MS, Sanders-Bush E, Conn PJ (2001) Metabotropic glutamate receptor 5-induced phosphorylation of extracellular signal-regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J Neurosci 21:9619–9628
Gill SS, Pulido OM (2001) Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol 29:208–223
Gill SS, Pulido OM, Mueller RW, McGuire PF (1999) Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res Bull 48:143–146
Iglesias I, Castillo CA, Leon D, Ruiz MA, Albasanz JL, Martin M (2007) Metabotropic glutamate receptor/phospholipase C system in female rat heart. Brain Res 1153:1–11. doi:10.1016/j.brainres.2007.01.010
Goldberg GS, Lampe PD, Nicholson BJ (1999) Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1:457–459. doi:10.1038/15693
Goldberg GS, Moreno AP, Lampe PD (2002) Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem 277:36725–36730. doi:10.1074/jbc.M109797200
Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Omori Y (1999) Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III 322:151–159
Jansen JA, van Veen TA, de Bakker JM, van Rijen HV (2010) Cardiac connexins and impulse propagation. J Mol Cell Cardiol 48:76–82. doi:10.1016/j.yjmcc.2009.08.018
Severs NJ, Bruce AF, Dupont E, Rothery S (2008) Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 80:9–19. doi:10.1093/cvr/cvn133
Laird DW (2005) Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 1711:172–182. doi:10.1016/j.bbamem.2004.09.009
Solan JL, Lampe PD (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419:261–272. doi:10.1042/BJ20082319
Lampe PD, Lau AF (2000) Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 384:205–215. doi:10.1006/abbi.2000.2131
Lampe PD, Lau AF (2004) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186. doi:10.1016/S1357-2725(03)00264-4
Lau AF, Kanemitsu MY, Kurata WE, Danesh S, Boynton AL (1992) Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell 3:865–874
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149:1503–1512
Opsahl H, Rivedal E (2000) Quantitative determination of gap junction intercellular communication by scrape loading and image analysis. Cell Adhes Commun 7:367–375
Solan JL, Lampe PD (2005) Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta 1711:154–163. doi:10.1016/j.bbamem.2004.09.013
Gu L, Liang X, Wang L, Yan Y, Ni Z, Dai H, Gao J, Mou S, Wang Q, Chen X, Wang L, Qian J (2012) Functional metabotropic glutamate receptors 1 and 5 are expressed in murine podocytes. Kidney Int 81:458–468. doi:10.1038/ki.2011.406
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–1362
Karim F, Wang CC, Gereau RWt (2001) Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci 21:3771–3779
Peavy RD, Conn PJ (1998) Phosphorylation of mitogen-activated protein kinase in cultured rat cortical glia by stimulation of metabotropic glutamate receptors. J Neurochem 71:603–612
Schinkmann KA, Kim TA, Avraham S (2000) Glutamate-stimulated activation of DNA synthesis via mitogen-activated protein kinase in primary astrocytes: involvement of protein kinase C and related adhesion focal tyrosine kinase. J Neurochem 74:1931–1940
Newton AC (1997) Regulation of protein kinase C. Curr Opin Cell Biol 9:161–167
Schonwasser DC, Marais RM, Marshall CJ, Parker PJ (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18:790–798
Sirnes S, Kjenseth A, Leithe E, Rivedal E (2009) Interplay between PKC and the MAP kinase pathway in Connexin43 phosphorylation and inhibition of gap junction intercellular communication. Biochem Biophys Res Commun 382:41–45. doi:10.1016/j.bbrc.2009.02.141
Doble BW, Ping P, Kardami E (2000) The epsilon subtype of protein kinase C is required for cardiomyocyte connexin-43 phosphorylation. Circ Res 86:293–301
Liao CK, Cheng HH, Wang SD, Yeih DF, Wang SM (2013) PKCvarepsilon mediates serine phosphorylation of connexin43 induced by lysophosphatidylcholine in neonatal rat cardiomyocytes. Toxicology 314:11–21. doi:10.1016/j.tox.2013.08.001
Leithe E, Rivedal E (2004) Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci 117:1211–1220. doi:10.1242/jcs.00951
Kanemitsu MY, Lau AF (1993) Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoylphorbol 13-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Mol Biol Cell 4:837–848
Catarino S, Ramalho JS, Marques C, Pereira P, Girao H (2011) Ubiquitin-mediated internalization of connexin43 is independent of the canonical endocytic tyrosine-sorting signal. Biochem J 437:255–267. doi:10.1042/BJ20102059
Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW (2003) Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem 278:30005–30014. doi:10.1074/jbc.M300614200
Rivedal E, Leithe E (2005) Connexin43 synthesis, phosphorylation, and degradation in regulation of transient inhibition of gap junction intercellular communication by the phorbol ester TPA in rat liver epithelial cells. Exp Cell Res 302:143–152. doi:10.1016/j.yexcr.2004.09.004
Brissette JL, Kumar NM, Gilula NB, Dotto GP (1991) The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol Cell Biol 11:5364–5371
Liang JY, Wang SM, Chung TH, Yang SH, Wu JC (2008) Effects of 18-glycyrrhetinic acid on serine 368 phosphorylation of connexin43 in rat neonatal cardiomyocytes. Cell Biol Int 32:1371–1379. doi:10.1016/j.cellbi.2008.08.007
Rivedal E, Opsahl H (2001) Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 22:1543–1550
Acknowledgments
This study was sponsored by the Natural Science Foundation of China (81270238), the Scientific Research Foundation for the Doctoral Degree, State Education Ministry of China (20100131110059) and supported by the Scientific Development Plan of Shandong Province of China (2012G0021850).
Conflict of interest
We do not have any competing interests in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, F., Yi, Sl., Hao, L. et al. Role of group I metabotropic glutamate receptors, mGluR1/mGluR5, in connexin43 phosphorylation and inhibition of gap junctional intercellular communication in H9c2 cardiomyoblast cells. Mol Cell Biochem 400, 213–222 (2015). https://doi.org/10.1007/s11010-014-2278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2278-x