Abstract
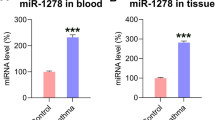
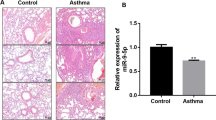
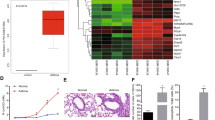
The pathological changes of airway smooth muscle (ASM) contribute to airway remodeling during asthma. Here, we investigated the effect of miR-145 on ASM function. We found that miR-145 was aberrantly more highly expressed in ASM cells exposed to cytokine stimulation that mimic the airway conditions of patients with asthma. Repression of miR-145 resulted in decreased ASM cell proliferation and migration in a dose-dependent manner and down-regulation of type I collagen and contractile protein MHC in ASM cells. qRT-PCR and Western blot analysis demonstrated that miR-145 negatively regulated the expression of downstream target Krüppel-like factor 4 (KLF4) protein, and overexpression of KLF4 attenuated the effects of miR-145 on ASM cells. Further studies showed that KLF4 significantly up-regulated the expression of p21 and down-regulated matrix metalloproteinase (MMP-2 and MMP-9). In conclusion, miR-145 overexpression in ASM cells significantly inhibited KLF4, and subsequently affected downstream p21, MMP-2, and MMP-9 expressions, eventually leading to enhanced proliferation and migration of ASM cells in vitro.






Similar content being viewed by others
References
Elias JA, Zhu Z, Chupp G, Homer RJ (1999) Airway remodeling in asthma. J Clin Invest 104:1001–1006
Busse W, Elias J, Sheppard D, Banks-Schlegel S (1999) Airway remodeling and repair. Am J Respir Crit Care Med 160:1035–1042
Elias JK (2000) Airway remodeling in asthma unanswered questions. Am J Respir Crit Care Med 161:S168–S171
Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M (2003) Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167:1360–1368
Kuhn AR, Schlauch K, Lao R, Halayko AJ, Gerthoffer WT, Singer CA (2010) MicroRNA expression in human airway smooth muscle cells: role of miR-25 in regulation of airway smooth muscle phenotype. Am J Respir Cell Mol Biol 42:506–513
Ji XY, Li JX, Xiang XD (2013) MicroRNAs: potential regulators of airway smooth muscle cell plasticity involved in asthma-induced airway remodeling. Asian Biomed 7:3–14
Goncharova EA, Lim PN, Chisolm A, Fogle HW III, Taylor JH, Goncharov DA, Eszterhas A, Panettieri RA Jr, Krymskaya VP (2010) Interferons modulate mitogen-induced protein synthesis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 299:L25–L35
Simon HU, Seelbach H, Ehmann R, Schmitz M (2003) Clinical and immunological effects of low-dose IFN-alpha treatment in patients with corticosteroid-resistant asthma. Allergy 58:1250–1255
Collison A, Mattes J, Plank M, Foster PS (2011) Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J Allergy Clin Immunol 128(160–167):e4
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang CX (2007) MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 100:1579–1588
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56–61
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460:705–710. doi:10.1038/nature08195
Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G (2009) The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 16:1590–1598
Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun L, Davis K, Weisel RD, Li RK (2014) Role of miR-145 in cardiac myofibroblast differentiation. J Mol Cell Cardiol 66:94–105
Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A (2011) Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem 286:28097–28110
Dahan D, Ekman M, Larsson-Callerfelt AK, Turczynska K, Boettger T, Braun T, Swärd K, Albinsson S (2014) Induction of angiotensin converting enzyme after miR-143/145 deletion is critical for impaired smooth muscle contractility. Am J Physiol Cell Physiol 00250:2014
Joshi SR, Comer BS, McLendon JM, Gerthoffer WT (2012) MicroRNA regulation of smooth muscle phenotype. Mol Cell Pharmacol 1:1–16
Clifford RL, Singer CA, John AE (2013) Epigenetics and miRNA emerge as key regulators of smooth muscle cell phenotype and function. Pulm Pharmacol Ther 26:75–85
Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, Arnould T, Deroanne C, Piette J, Cataldo D, Colige A (2011) MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One 6:e16509
Cushing L, Kuang P, Fine A, Shao F, Cardoso W, Little FF, Lu J (2011) Mir-29 regulation of airway remodeling in asthma. Am J Respir Crit Care Med 183:A4054
Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA Jr, Amrani Y (2003) Tumor necrosis factor alpha modulates airway smooth muscle function via the autocrine action of interferon beta. J Biol Chem 278:50615–50623
Amrani Y, Tliba O, Choubey D, Huang CD, Krymskaya VP, Eszterhas A, Lazaar AL, Panettieri RA (2003) IFN-γ inhibits human airway smooth muscle cell proliferation by modulating the E2F-1/Rb pathway. Am J Physiol Lung Cell Mol Physiol 284:L1063–L1071
Autieri MV (2008) Kruppel-like factor 4: transcriptional regulator of proliferation, or inflammation, or differentiation, or all three? Circ Res 102:1455–1467
An J, Golech S, Klaewsongkram J, Zhang Y, Subedi K, Huston GE, Wood WH, Wersto RP, Becker KG, Swain SL, Weng N (2011) Kruppel-like factor 4 (KLF4) directly regulates proliferation in thymocyte development and IL-17 expression during Th17 differentiation. FASEB J 25:3634–3645
Yoshida T, Kaestner KH, Owens GK (2008) Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 102:1548–1557
Wassmann S, Wassmann K, Jung A, Velten M, Knuefermann P, Petoumenos V, Becher U, Werner C, Mueller C, Nickenig G (2007) Induction of p53 by GKLF is essential for inhibition of proliferation of vascular smooth muscle cells. J Mol Cell Cardiol 43:301–307
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y, Song C, Zhu S, Leng Y, Wang G, Chen W, Du P, Huang S, Zhou X, Kang J, Cui L (2014) MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer 110:450–458
Mohan N, Ai W, Chakrabarti M, Banik NL, Ray SK (2013) KLF4 overexpression and apigenin treatment down regulated anti-apoptotic Bcl-2 proteins and matrix metalloproteinases to control growth of human malignant neuroblastoma SK-N-DZ and IMR-32 cells. Mol Oncol 7:464–474
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (No. 81102252).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest concerning this article.
Rights and permissions
About this article
Cite this article
Liu, Y., Sun, X., Wu, Y. et al. Effects of miRNA-145 on airway smooth muscle cells function. Mol Cell Biochem 409, 135–143 (2015). https://doi.org/10.1007/s11010-015-2519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2519-7