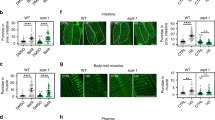
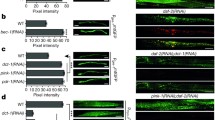
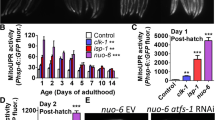
Abstract
Autophagy of mitochondria, i.e., mitophagy, plays a crucial role in coping with stressors in the aging process, metabolic disturbances, and neurological disorders. Impairments of the process might consequently lead to enhanced accumulation of aged and aggregated proteins and reduced cellular integrity in response to stress. In the present study, we used the stress-sensitive mutant mev-1 of Caenorhabditis elegans to assess the effects of the knockdown of mitophagy relevant genes on survival under heat stress, the amount of autophagosomes, and on protein aggregation. RNA interference for dct-1, drp-1, eat-3, fis-1, fzo1, glb-1, pink-1, and pgam-5 all resulted in a significant reduction of survival time at 37 °C. These effects were associated with a decrease in autophagosomal flux of proteins, as indicated by increased accumulation of GFP-tagged SQST-1, and a reduced amount of lysosomes demonstrating that autophagy was hampered. Moreover, the gene knockdowns led to increased levels of reactive oxygen species in mitochondria and an enhanced protein aggregation. In conclusion, our studies show that mitophagy is of central importance to keep mitochondria functional in order to prevent production of excess reactive oxygen species and protein aggregation and finally a reduction of survival under heat stress.





Similar content being viewed by others
References
Kauppila TES, Kauppila JHK, Larsson N-G (2017) Mammalian mitochondria and aging: an update. Cell Metab 25(1):57–71. https://doi.org/10.1016/j.cmet.2016.09.017
Akhter F, Chen D, Yan SF et al (2017) Mitochondrial perturbation in Alzheimer’s disease and diabetes. Prog Mol Biol Transl Sci 146:341–361. https://doi.org/10.1016/bs.pmbts.2016.12.019
Barja G (2014) The mitochondrial free radical theory of aging. Prog Mol Biol Transl Sci 127:1–27. https://doi.org/10.1016/B978-0-12-394625-6.00001-5
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. https://doi.org/10.1038/cdd.2012.81
Arduíno DM, Esteves AR, Cardoso SM (2011) Mitochondrial fusion/fission, transport and autophagy in Parkinson’s disease: when mitochondria get nasty. Parkinsons Dis 2011:767230. https://doi.org/10.4061/2011/767230
Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18(R2):R169–R176. https://doi.org/10.1093/hmg/ddp326
Olzmann JA, Li L, Chin LS (2008) Aggresome formation and neurodegenerative diseases: therapeutic implications. Curr Med Chem 15(1):47–60
Park J, Park Y, Ryu I et al (2017) Misfolded polypeptides are selectively recognized and transported toward aggresomes by a CED complex. Nat Commun 8:15730. https://doi.org/10.1038/ncomms15730
Park Y, Park J, Kim YK (2017) Crosstalk between translation and the aggresome-autophagy pathway. Autophagy. https://doi.org/10.1080/15548627.2017.1358849
Zaarur N, Meriin AB, Bejarano E et al (2014) Proteasome failure promotes positioning of lysosomes around the aggresome via local block of microtubule-dependent transport. Mol Cell Biol 34(7):1336–1348. https://doi.org/10.1128/MCB.00103-14
Stürner E, Behl C (2017) The role of the multifunctional BAG3 protein in cellular protein quality control and in disease. Front Mol Neurosci 10:177. https://doi.org/10.3389/fnmol.2017.00177
Shi Y, Williamson G (2015) Comparison of the urinary excretion of quercetin glycosides from red onion and aglycone from dietary supplements in healthy subjects: a randomized, single-blinded, cross-over study. Food Funct 6(5):1443–1448. https://doi.org/10.1039/c5fo00155b
Hyttinen JMT, Amadio M, Viiri J et al (2014) Clearance of misfolded and aggregated proteins by aggrephagy and implications for aggregation diseases. Ageing Res Rev 18:16–28. https://doi.org/10.1016/j.arr.2014.07.002
Liu H, Dai C, Fan Y et al (2017) From autophagy to mitophagy: the roles of P62 in neurodegenerative diseases. J Bioenerg Biomembr 49(5):413–422. https://doi.org/10.1007/s10863-017-9727-7
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94
Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263(1–2):103–112
Lehner B, Tischler J, Fraser AG (2006) RNAi screens in Caenorhabditis elegans in a 96-well liquid format and their application to the systematic identification of genetic interactions. Nat Protoc 1(3):1617–1620. https://doi.org/10.1038/nprot.2006.245
Fitzenberger E, Boll M, Wenzel U (2013) Impairment of the proteasome is crucial for glucose-induced lifespan reduction in the mev-1 mutant of Caenorhabditis elegans. Biochim Biophys Acta 1832(4):565–573. https://doi.org/10.1016/j.bbadis.2013.01.012
Gill MS, Olsen A, Sampayo JN et al (2003) An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radic Biol Med 35(6):558–565. https://doi.org/10.1016/S0891-5849(03)00328-9
Schiavi A, Maglioni S, Palikaras K et al (2015) Iron-Starvation-induced mitophagy mediates lifespan extension upon mitochondrial stress in C. elegans. Curr Biol 25(14):1810–1822. https://doi.org/10.1016/j.cub.2015.05.059
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407. https://doi.org/10.1146/annurev.genet.39.110304.095751
Zhu J, Wang KZQ, Chu CT (2013) After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 9(11):1663–1676. https://doi.org/10.4161/auto.24135
Balch WE, Sznajder JI, Budinger S et al (2014) Malfolded protein structure and proteostasis in lung diseases. Am J Respir Crit Care Med 189(1):96–103. https://doi.org/10.1164/rccm.201306-1164WS
Palikaras K, Daskalaki I, Markaki M et al (2017) Mitophagy and age-related pathologies: development of new therapeutics by targeting mitochondrial turnover. Pharmacol Ther 178:157–174. https://doi.org/10.1016/j.pharmthera.2017.04.005
Jimenez RE, Kubli DA, Gustafsson ÅB (2014) Autophagy and mitophagy in the myocardium: therapeutic potential and concerns. Br J Pharmacol 171(8):1907–1916. https://doi.org/10.1111/bph.12477
Palikaras K, Tavernarakis N (2012) Mitophagy in neurodegeneration and aging. Front Genet 3:297. https://doi.org/10.3389/fgene.2012.00297
Heo J-m, Ordureau A, Paulo JA et al (2015) The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell 60(1):7–20. https://doi.org/10.1016/j.molcel.2015.08.016
Frank M, Duvezin-Caubet S, Koob S et al (2012) Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 1823(12):2297–2310. https://doi.org/10.1016/j.bbamcr.2012.08.007
Rolland SG, Lu Y, David CN et al (2009) The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. J Cell Biol 186(4):525–540. https://doi.org/10.1083/jcb.200905070
Cohen MM, Amiott EA, Day AR et al (2011) Sequential requirements for the GTPase domain of the mitofusin Fzo1 and the ubiquitin ligase SCFMdm30 in mitochondrial outer membrane fusion. J Cell Sci 124(Pt 9):1403–1410. https://doi.org/10.1242/jcs.079293
Gomes LC, Scorrano L (2008) High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta 1777(7–8):860–866. https://doi.org/10.1016/j.bbabio.2008.05.442
Frank S, Gaume B, Bergmann-Leitner ES et al (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1(4):515–525. https://doi.org/10.1016/S1534-5807(01)00055-7
Palikaras K, Lionaki E, Tavernarakis N (2015) Coupling mitogenesis and mitophagy for longevity. Autophagy 11(8):1428–1430. https://doi.org/10.1080/15548627.2015.1061172
Lakowski B, Hekimi S (1998) The genetics of caloric restriction in Caenorhabditis elegans. Proc Nat Acad Sci 95(22): 13091–13096
Dainin K, Ide R, Maeda A et al (2017) Pyridoxamine scavenges protein carbonyls and inhibits protein aggregation in oxidative stress-induced human HepG2 hepatocytes. Biochem Biophys Res Commun 486(3):845–851. https://doi.org/10.1016/j.bbrc.2017.03.147
Wu Y, Luo Y (2005) Transgenic C. elegans as a model in Alzheimer’s research. Curr Alzheimer Res 2(1):37–45
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit organizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Civelek, M., Mehrkens, JF., Carstens, NM. et al. Inhibition of mitophagy decreases survival of Caenorhabditis elegans by increasing protein aggregation. Mol Cell Biochem 452, 123–131 (2019). https://doi.org/10.1007/s11010-018-3418-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3418-5