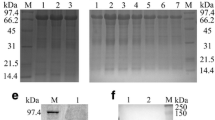
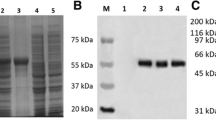
Abstract
Using a combination of bioinformation analysis and Dot blot technique, a gene, designated hereafter as the duck enteritis virus (DEV) UL31 gene (GenBank accession number EF643559), was identified from the DEV CHv genomic library. Then, the UL31 gene was cloned and sequenced, which was composed of 933 nucleotides encoding 310 amino acids. Multiple sequence alignment suggested that the UL31 gene was highly conserved in Alphaherpesvirinae and similar to the other herpesviral UL31. Phylogenetic analysis showed that the gene had a close evolutionary relationship with the avian herperviruses, and DEV should be placed into a single cluster within the subfamily Alphaherpesvirinae. Antigen prediction indicated that several potential B-cell epitopes sites located in the UL31 protein. To further study, the UL31 gene was cloned into a pET prokaryotic expression vector and transformed into Escherichia coli BL21 (DE3). A 55 kDa fusion protein was induced by the further culture at 37°C after addition of 0.8 mM IPTG. Polyclonal antibody raised against the recombinant UL31 from rabbit was prepared. A protein about 55 kDa that reacted with the antibody was detected in immunoblots of bacterial proteins, suggesting that the 55 kDa protein was the product of the UL31 gene. Immunofluorescence analysis revealed that the protein was localized in very fine punctate forms in the nuclei of infected cells. Our results may provide some insight for further research about the gene and also enrich the database of herpesvirus.








Similar content being viewed by others
Abbreviations
- DEV:
-
Duck enteritis virus
- HSV-1:
-
Herpes simplex virus 1
- HCMV:
-
Human cytomegalovirus
- EBV:
-
Epstein-barr virus
- DVE:
-
Duck viral enteritis
- HHV-3:
-
Human herpesvirus 3
- MDV-1:
-
Marek’s disease virus type 1
- CeHV-9:
-
Cercopithecine herpesvirus 9
- MeHV-1:
-
Meleagrid herpesvirus 1
- EHV-1:
-
Equid herpesvirus 1
- BoHV-5:
-
Bovine herpesvirus 5
- MDV-2:
-
Marek’s disease virus type 2
- ILTV:
-
Infection laryngotracheitis virus
- CeHV-16:
-
Cercopithecine herpesvirus 16
- HHV-2:
-
Human herpesvirus 2
- BoHV-1:
-
Bovine herpesvirus 1
- CeHV-1:
-
Cercopithecine herpesvirus 1
- SuHV-1:
-
Suid herpesvirus 1
- HHV-1:
-
Human herpesvirus 1
- HHV-7:
-
Human herpesvirus 7
- PCMV:
-
Porcine cytomegalovirus
- MCMV:
-
Murine cytomegalovirus
- HHV-6:
-
Human herpesvirus 6
- HHV-5:
-
Human herpesvirus 5
- HHV-8:
-
Human herpesvirus 8
- BoHV-4:
-
Bovine herpesvirus 4
- SaHV-2:
-
Saimiriine herpesvirus 2
- EHV-2:
-
Equid herpesvirus 2
- MDPV:
-
Muscovy duck parvovirus
- Prv:
-
Pseudorabies virus
- Gpv:
-
Goose parvovirus
References
Roizman B, Pellett PE (2001) The family herpesviridae: a brief introduction. Field Virol 2:2381–2397
Park R, Baines JD (2006) Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J Virol 80:494–504. doi:10.1128/JVI.80.1.494-504.2006
Simpson-Holley M, Colgrove RC, Nalepa G et al (2005) Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J Virol 79:12840–12851. doi:10.1128/JVI.79.20.12840-12851.2005
Chang PC, Hsieh ML, Shien JH et al (2001) Complete nucleotide sequence of avian paramyxovirus type 6 isolated from ducks. J Gen Virol 82:2157–2168
Dal Monte P, Pignatelli S, Zini N (2002) Analysis of intracellular and intraviral localization of the human cytomegalovirus UL53 protein. J Gen Virol 83:1005–1012
Gonnella R, Farina A, Santarelli R et al (2005) Characterization and intracellular localization of the epstein-barr virus protein BFLF2: interactions with BFRF1 and with the nuclear lamina. J Virol 79:3713–3727. doi:10.1128/JVI.79.6.3713-3727.2005
Lake CM, Hutt-Fletcher LM (2004) The epstein-barr virus BFRF1 and BFLF2 proteins interact and coexpression alters their cellular localization. Virology 320:99–106. doi:10.1016/j.virol.2003.11.018
Simpson-Holley M, Baines J, Roller R et al (2004) Herpes simplex virus 1 U(L)31 and U(L)34 gene products promote the late maturation of viral replication compartments to the nuclear periphery. J Virol 78:5591–5600. doi:10.1128/JVI.78.11.5591-5600.2004
Reynolds AE, Liang L, Baines JD (2004) Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J Virol 78:5564–5575. doi:10.1128/JVI.78.11.5564-5575.2004
Zhu HY, Yamada H, Jiang YM et al (1999) Intracellular localization of the UL31 protein of herpes simplex virus type 2. Arch Virol 144:1923–1935. doi:10.1007/s007050050715
Klupp BG, Granzow H, Fuchs W et al (2007) Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc Natl Acad Sci USA 104:7241–7246. doi:10.1073/pnas.0701757104
Helferich D, Veits J, Mettenleiter TC et al (2007) Identification of transcripts and protein products of the UL31, UL37, UL46, UL47, UL48, UL49 and US4 gene homologues of avian infectious laryngotracheitis virus. J Gen Virol 88:719–731. doi:10.1099/vir.0.82532-0
Mou F, Forest T, Baines JD (2007) US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol 81:6459–6470. doi:10.1128/JVI.00380-07
Schnee M, Ruzsics Z, Bubeck A et al (2006) Common and specific properties of herpesvirus UL34/UL31 protein family members revealed by protein complementation assay. J Virol 80:11658–11666. doi:10.1128/JVI.01662-06
Davison S, Converse KA, Hamir AN et al (1993) Duck viral enteritis in Muscovy ducks in Pennsylvania. Avian Dis 37:1142–1146. doi:10.2307/1591927
Montali RJ, Bush M, Greenwell GA (1976) An epornitic of duck viral enteritis in a zoological park. J Am Vet Med Assoc 169:954–958
Proctor SJ (1976) Pathogenesis of duck plague in the bursa of fabricius, thymus, and spleen. Am J Vet Res 37:427–431
Baudet AE (1923) Mortality in ducks in the Netherlands caused by a filterable virus: fowl plague. Tijdschr Diergeneeskd 55:455–459
Leibovitz L, Hwang J (1968) Duck plague on the American continent. Avian Dis 12:361–378. doi:10.2307/1588237
Jansen J, Kunst H (1964) The reported incidence of duck plague in Europe and Asia. Tijdschr Diergeneeskd 89:765–769
Lucam F (1949) La peste aviaire en France. Repoc 14th Int Vet Congr 2:380–382
Saif YM, Barnes HJ, Glisson JR et al (2003) Diseases of poultry, 11th edn. Lowa State University Press, Ames
Fauquet CM, Mayo MA, Maniloff J (2005) Virus taxonomy: eighth report of the international committee on taxonomy of viruses. Elsevier Academic Press, California
Gardner R, Wilkerson J, Johnson JC (1993) Molecular characterization of the DNA of Anatid herpesvirus 1. Intervirology 36:99–112
Cheng AC, Wang MS, Wen M (2006) Construction of duck enteritis virus gene libraries and discovery, cloning and identification of viral nucleocapsid protein gene. High Technol Lett 16:948–953
Oraveerakul K, Choi CS, Molitor TW (1990) Detection of porcine parvovirus using nonradioactive nucleic acid hybridization. J Vet Diagn Invest 2:85–91
Altschul SF, Madden TL, Schaffer AA et al (1997) A new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Bendtsen JD, Nielsen H, von Heijne G et al (2004) Improved prediction of signal peptides: signalP 3.0. J Mol Biol 340:783–795. doi:10.1016/j.jmb.2004.05.028
Higgins DG, Bleasby AJ, Fuchs R (2002) CLUSTAL V: improved software for multiple sequence alignment. Oxford University Press, Oxford
Higgins DG (1994) CLUSTAL V: multiple alignment of DNA and protein sequences. Methods Mol Biol 25:307–318. doi:10.1385/0-89603-276-0:307
Page RDM (1996) Tree view: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Burland TG (2000) DNASTAR’s Lasergene sequence analysis software. Methods Mol Biol 132:71–91
Van Regenmortel MH (1992) Protein antigenicity. Mol Biol Rep 16:133–138. doi:10.1007/BF00464700
Xie W, Cheng AC, Wang MS, Chang H et al (2009) Expression and characterization of the UL31 protein from Duck enteritis virus. Virol J 6:19. doi:10.1186/1743-422x-6-9
Towbin H, Staehelin T, Gordin J (1976) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354. doi:10.1073/pnas.76.9.4350
Leary JJ, Brigati DJ, Ward DC (1983) Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: bio-blots. Proc Natl Acad Sci USA 80:4045–4049. doi:10.1073/pnas.80.13.4045
Chang YE, Van Sant C, Krug PW (1997) The null mutant of the U(L)31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol 11:8307–8315
Klupp BG, Granzow H, Fuchs W (2004) Pseudorabies virus UL3 gene codes for a nuclear protein which is dispensable for viral replication. J Virol 1:464–4672
Fuchs W, Klupp BG, Mettenleiter TC (2002) The interacting UL31 and UL34 gene products of Pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. Virology 76:364–378. doi:10.1128/JVI.76.1.364-378.2002
Mark L, Zsolt R, Ulrich HK (2006) Functional domains of murine cytomegalovirus nuclear egress protein M53/p38. J Virol 80:73–84. doi:10.1128/JVI.80.1.73-84.2006
McGeoch DJ, Cook S (1994) Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescal. J Mol Biol 238:9–22. doi:10.1006/jmbi.1994.1264
Bjerke SL, Roller RJ (2006) Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 347:261–276. doi:10.1016/j.virol.2005.11.053
Reynolds AE, Ryckman BJ, Baines JD (2001) U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol 75:8803–8817. doi:10.1128/JVI.75.18.8803-8817.2001
Yamauchi Y, Shiba C, Goshima F, Nawa A (2001) Herpes simplex virus type 2 UL34 protein requires UL31 protein for its relocation to the internal nuclear membrane in transfected cells. J Gen Virol 82:1423–1428
Hopp TP, Woods KR (1981) Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA 78:3824–3828. doi:10.1073/pnas.78.6.3824
Welling GW, Weijer WJ (1985) Prediction of sequential antigenic regions in proteins. FEBS Lett 188:215–218. doi:10.1016/0014-5793(85)80374-4
Acknowledgments
The research was supported by the National Natural Science Foundation of China (30771598), Changjiang Scholars and Innovative Research Team in University(IRT0848), the earmarked fund for Modern Agro-industry Technology Research System (2009–2013), New Century Excellent Talents Program in University (NCET-06-0818), the Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China (706050), the Cultivation Fund of the Key Scientific and Technical Innovation Project, department of Education of Sichuan Province (07ZZ028), Sichuan Province Outstanding Youths Fund (07ZQ026-132), and Sichuan Province Basic Research Program (07JY029-016/07JY029-017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, W., Cheng, A., Wang, M. et al. Molecular cloning and characterization of the UL31 gene from Duck enteritis virus. Mol Biol Rep 37, 1495–1503 (2010). https://doi.org/10.1007/s11033-009-9546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9546-y