Abstract
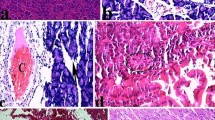
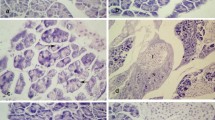
In order to investigate an association between alcohol consumption and lysosomal cysteine protease induced pancreatic injury and preventive effect of gallic acid as dose-dependent, we determined myeloperoxidase and malondialdehyde levels, serum amylase activities and cathepsin B and L activities in the cytosolic and lysosomal fractions of pancreatic tissue in the ethanol (8 g/kg) and ethanol plus gallic acid (at different doses 50, 100 and 200 mg/kg) given rats. Absolute ethanol (8 g/kg) was given by oral gavage. Gallic acid was dissolved in the saline (2 ml/kg) and administered before 30 min the oral administration of ethanol. Pancreatic myeloperoxidase and also malondialdehyde levels and serum amylase activities were measured. Besides, histological investigations were made. Cathepsin B activities in the cytosolic fraction were decreased by gallic acid (200 mg/kg) and increased in ethanol given rats. Cytosolic/lysosomal ratio of cathepsin B and L were found to be low in the all doses of gallic acid as compared to ethanol group. Serum amylase, pancreatic myeloperoxidase activities and malondialdehyde levels in the ethanol group were higher than in the control group. These were not statistically significant for myeloperoxidase and malondialdehyde. Also, our histopathologic results indicated that ethanol administration increased pancreatic tissue injury. Gallic acid especially at 200 mg/kg improved ethanol-mediated pancreatic tissue damage.In conclusion, gallic acid treatments were decreased release of lysosomal cathepsin B and L enzymes into cytoplasmic fraction and prevented alcohol mediated pancreatic tissue injury. Preventive effect of gallic acid might be dose-dependent.














Similar content being viewed by others
Abbreviations
- MPO:
-
Myeloperoxidase
- MDA:
-
Malondialdehyde
- FAEE:
-
Fatty acid ethyl esters
- C:
-
Cytosolic
- L:
-
Lysosomal
- C/L:
-
Cytosolic/lysosomal
- FEL:
-
Fractions enriched with lysosomes
- SPSS:
-
Statistical package for social sciences
- CYP2E1:
-
Cytochrome P4502E1
- PMN:
-
Polymorphonuclear
References
Wilson JS, Apte MV (2003) Role of alcohol metabolism in alcoholic pancreatitis. Pancreas 27:311–315
Pandol SJ (2005) Acute pancreatitis. Curr Opin Gastroenterol 21:538–543
Acker JDG, Saluja AK, Bhagat L, Singh VP, Song AM, Ster ML (2002) Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatititis severity. Am J Physiol Gastrointest Liver Physiol 283:794–800
Prince MSP, Priscilla H, Devika PT (2009) Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in wistar rats. Eur J Pharmacol 139:139–143
Işlekel H, Işlekel S, Güner G, Ozdamar N (1999) Evaluation of lipid peroxidation, cathepsin L and acid phosphatase activities in experimental brain ischemia–reperfusion. Brain Res 843:18–24
Conus S, Simon HU (2008) Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol 76:1374–1382
Karthikeyan K, SaralaBai BR, Niranjali DS (2007) Grape seed prothocyanidins ameliorates isoproterenol induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes: an in vivi study. Life Sci 81:11615–11621
Shahidi F, Naczk M (2004) Phenolics in food and nutraceuticals. CRC Press, Boca Raton, p 564
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. Am J Clin Nutr 81:230–242
Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J (2007) Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food ChemToxicol 45:328–336
Ichihara K, Haneda T, Onodera S, Abiko Y (1987) Inhibition of ischemia-induced subcellular redistribution of lysosomal enzymes in the perfused rat heart by the calcium entry blocker, diltiazem. J Pharmacol Exp Ther 242:1109–1113
Kirschke H, Wood L, Reisen F (1983) Activity of lysosomal cysteine proteinase during differentiation of rat skeletal muscle. Biochem J 214:871–877
Ekingen G, Ceran C, Demirtola A, Demiroğulları B, Sancak B, Poyraz A, Sönmez K, Basaklar AC, Kale N (2006) The effect of reperfusion duration in intestinal ischemia-reperfusion on biochemical parameters and small intestinal anastomosis healing. J Inonu University Med Fac 13:7–12
Werner J, Saghır M, Warshaw AL, Lewandrowskı KB, Laposata M, Lozzo RV, Carter EA, Schatz RJ, Ferna C, Castillo ND (2002) Alcoholic pancreatitis in rats: injury from nonoxidative metabolites of ethanol. Jens Am J Physiol Gastrointest Liver Physiol 283:65–73
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Layne E (1957) Spectrophotometric and turbidimetric methods for measuring proteins. Method Enzymol 10:447–455
Estival A, Clemente F, Ribet A (1981) Ethanol metabolism by the rat pancreas. Toxicol Appl Pharmacol 61:155–165
Hamamoto T, Yamada S, Hirayama C (1990) Nonoxidative metabolism of ethanol in the pancreas; implication in alcoholic pancreatic damage. Biochem Pharmacol 39:241–245
Haber PS, Apte MV (1993) Fatty acid ethyl esters increase rat pancreatic lysosomal fragility. J Lab Clin Med 121:759–764
Werner J, Laposata M, Fernandez-del CC (1997) Pancreatic injury in rats induced by fatty ethyl ester, a non-oxidative metabolite of alcohol. Gastroenterology 113:286–294
Byung KP, Chung JB, Lee JH, Suh JH, Park SW, Song SY, Hyeyoung K, Kyung HK, Kang JK (2003) Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol 9:2266–2269
Reynolds LD, Wilson NG. Scribes and Scholars. 3rd Ed. Oxford pp 193-4, 1991
Monach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenol: food sources and bioavailability. Am J Clin Nutr 79:727–747
Conflict of interest
The authors have declared no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanbak, G., Canbek, M., Oğlakçı, A. et al. Preventive role of gallic acid on alcohol dependent and cysteine protease-mediated pancreas injury. Mol Biol Rep 39, 10249–10255 (2012). https://doi.org/10.1007/s11033-012-1901-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1901-8