Abstract
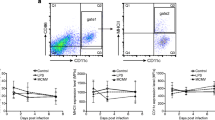
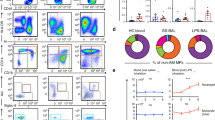
Respiratory dendritic cells (DCs), especially conventional DCs (cDCs), are critically involved in the induction phase of the immune response in the respiratory system. However, little information concerning cDC kinetics in acute lung injury (ALI) is available. In this study, we have used a murine model of LPS-induced ALI to examine the kinetics and phenotype of respiratory, circulating and splenic cDCs. cDCs in the lung, blood, and spleen and the IL-6 level in the lung were detected at 6, 12 and 24 h after PBS or LPS challenge. In the ALI group, a rapid cDCs accumulation in the lung was observed, and there were highly significant correlations between the frequency of respiratory cDCs or the percentage of cDC expressing CD80 and the IL-6 concentration. However, the frequency of peripheral blood cDCs decreased rapidly in ALI mice, followed by a marked expansion. In addition, splenic cDCs only showed a transient expansion in ALI. cDCs within the blood, lungs and spleens had undergone a modest maturation in the ALI group. Our findings demonstrate that LPS-induced ALI provokes a dynamic and distinct distribution as well as phenotype changes in pulmonary, circulatory and splenic cDC populations. Lung cDCs may participate in the early inflammatory response to ALI.





Similar content being viewed by others
References
Randolph GJ, Ochando J, Partida-Sanchez S (2008) Migration of dendritic cell subsets and their precursors. Annu Rev Immunol 26:293–316
Iwasaki A (2007) Mucosal dendritic cells. Annu Rev Immunol 25:381–418
Vermaelen K, Pauwels R (2005) Pulmonary dendritic cells. Am J Respir Crit Care Med 172:530–551
de Heer HJ, Hammad H, Kool M, Lambrecht BN (2005) Dendritic cell subsets and immune regulation in the lung. Semin Immunol 17:295–303
Beaty SR, Rose CE Jr, Sung SS (2007) Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 178:1882–1895
Zhang H, Slutsky AS (2009) Year in review 2008: critical care–respirology. Crit Care 13:225
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693
Efron P, Moldawer LL (2003) Sepsis and the dendritic cell. Shock 20:386–401
Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C (2009) Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care 13:R119
Hotchkiss RS, Opal S (2010) Immunotherapy for sepsis–a new approach against an ancient foe. N Engl J Med 363:87–89
Grimaldi D, Louis S, Pene F, Sirgo G, Rousseau C, Claessens YE, Vimeux L, Cariou A, Mira JP, Hosmalin A et al (2011) Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med 37:1438–1446
Wang Y, Bi Y, Wu K, Wang C (2011) Dendritic cell co-transfected with FasL and allergen genes induces T cell tolerance and decreases airway inflammation in allergen induced murine model. Mol Biol Rep 38:809–817
Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, Soler P (2010) A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 182:385–395
Liu J, Zhang PS, Yu Q, Liu L, Yang Y, Guo FM, Qiu HB (2012) Losartan inhibits conventional dendritic cell maturation and Th1 and Th17 polarization responses: novel mechanisms of preventive effects on lipopolysaccharide-induced acute lung injury. Int J Mol Med 29:269–276
Osterholzer JJ, Ames T, Polak T, Sonstein J, Moore BB, Chensue SW, Toews GB, Curtis JL (2005) CCR2 and CCR6, but not endothelial selectins, mediate the accumulation of immature dendritic cells within the lungs of mice in response to particulate antigen. J Immunol 175:874–883
Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE (2002) Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol 168:2493–2500
MacPherson GG, Jenkins CD, Stein MJ, Edwards C (1995) Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J Immunol 154:1317–1322
De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M (1996) Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med 184:1413–1424
Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, Kojima K, Shimokata K, Hasegawa Y (2004) CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol 30:808–815
Smith KM, Mrozek JD, Simonton SC, Bing DR, Meyers PA, Connett JE, Mammel MC (1997) Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit Care Med 25:1888–1897
Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, Joetham A, Park JW, Dakhama A, Gelfand EW (2006) Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med 173:42–55
Cleret A, Quesnel-Hellmann A, Vallon-Eberhard A, Verrier B, Jung S, Vidal D, Mathieu J, Tournier JN (2007) Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J Immunol 178:7994–8001
Anis MM, Fulton SA, Reba SM, Liu Y, Harding CV, Boom WH (2008) Modulation of pulmonary dendritic cell function during mycobacterial infection. Infect Immun 76:671–677
Martins GA, Da Gloria Da Costa Carvalho M, Rocha Gattass C (2003) Sepsis: a follow-up of cytokine production in different phases of septic patients. Int J Mol Med 11:585–591
Biancone L, Cantaluppi V, Camussi G (1999) CD40-CD154 interaction in experimental and human disease (review). Int J Mol Med 3:343–353
Veckman V, Julkunen I (2008) Streptococcus pyogenes activates human plasmacytoid and myeloid dendritic cells. J Leukoc Biol 83:296–304
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:L379–L399
Shinohara ML, Cantor H (2007) The bridge between dendritic cells and asthma. Nat Med 13:536–538
Mizgerd JP (2008) Acute lower respiratory tract infection. N Engl J Med 358:716–727
von Wulffen W, Steinmueller M, Herold S, Marsh LM, Bulau P, Seeger W, Welte T, Lohmeyer J, Maus UA (2007) Lung dendritic cells elicited by Fms-like tyrosine 3-kinase ligand amplify the lung inflammatory response to lipopolysaccharide. Am J Respir Crit Care Med 176:892–901
Legge KL, Braciale TJ (2003) Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18:265–277
van Rijt LS, Prins JB, Leenen PJ, Thielemans K, de Vries VC, Hoogsteden HC, Lambrecht BN (2002) Allergen-induced accumulation of airway dendritic cells is supported by an increase in CD31hiLy-6Cneg bone marrow precursors in a mouse model of asthma. Blood 100:3663–3671
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81070049, No. 81170057) and Project of national key clinical specialty construction of China (2100299) and Health research special funds for public welfare projects (201202011) and the Scientific and Technological Research Projects of Suzhou City (SYS201251). We also thank Profs. Wang Li-Xing, Zhang Jian-Qiong and Shen Chuan-Lai (Southeast University School of Medicine, Nanjing) for their insightful suggestion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Zhang, PS., Yu, Q. et al. Kinetic and distinct distribution of conventional dendritic cells in the early phase of lipopolysaccharide-induced acute lung injury. Mol Biol Rep 39, 10421–10431 (2012). https://doi.org/10.1007/s11033-012-1921-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1921-4