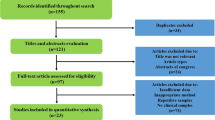
Abstract
Epidemiological studies have reported the relationship between vacuolating cytotoxin A (vacA) s-/m- region genotypes and duodenal ulcer (DU), but the results remained inconclusive. We performed the present meta-analysis to investigate a more authentic association between vacA s-/m- region genotypes and DU. Literature search was performed by searching Embase, PubMed and ISI Web of Science databases as well as checking references from identified articles, reviews and the abstracts presented at related scientific societies meetings. The association was assessed by combined odds ratio (OR) with 95 % confidence interval (CI). A total of 42 studies were included in our final meta-analysis. The combined ORs (95 % CIs) showed that vacA s1 (OR = 2.96, 95 % CI = 2.34–3.75), m1 (OR = 1.46, 95 % CI = 1.05–2.04) and s1m1 (OR = 1.89, 95 % CI = 1.47–2.42) were associated with increased DU risk significantly in the overall studied population. Subgroup analyses by ethnicity showed that vacA s1 increased the risk of DU in Asian countries (OR = 1.92, 95 % CI = 1.30–2.83), European countries (OR = 3.58, 95 % CI = 2.13–6.03) and Latin American countries (OR = 4.20, 95 % CI = 2.21–7.98); vacA m1 increased the risk of DU in Latin American countries (OR = 2.98, 95 % CI = 1.59–5.56); vacA s1m1 increased the risk of DU in Asian countries (OR = 2.04, 95 % CI = 1.12–3.73) and Latin American countries (OR = 2.05, 95 % CI = 1.20–3.48); vacA s2m1 increased the risk of DU in Latin American countries (OR = 2.30, 95 % CI = 1.17–4.50). The data suggest that genotype testing of vacA s- and m- region will be useful in screening susceptible individuals for DU development.








Similar content being viewed by others
References
Piper DW, Nasiry R, McIntosh J, Shy CM, Pierce J, Byth K (1984) Smoking, alcohol, analgesics, and chronic duodenal ulcer. A controlled study of habits before first symptoms and before diagnosis. Scand J Gastroenterol 19(8):1015–1021
Zapata-Colindres JC, Zepeda-Gomez S, Montano-Loza A, Vazquez-Ballesteros E, de Jesus Villalobos J, Valdovinos-Andraca F (2006) The association of Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs in peptic ulcer disease. Canadian journal of gastroenterology = Journal canadien de gastroenterologie 20 (4):277-280
NIH Consensus Conference (1994) Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA, J Am Med Assoc 272(1):65–69
Go MF (2002) Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther 16(Suppl 1):3–15
Cover TL (1996) The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol 20(2):241–246
Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL (1995) Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 270(30):17771–17777
Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC (2007) A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133(3):926–936. doi:10.1053/j.gastro.2007.06.056
Basso D, Navaglia F, Brigato L, Piva MG, Toma A, Greco E, Di Mario F, Galeotti F, Roveroni G, Corsini A, Plebani M (1998) Analysis of Helicobacter pylori vacA and cagA genotypes and serum antibody profile in benign and malignant gastroduodenal diseases. Gut 43(2):182–186
Go MF, Cissell L, Graham DY (1998) Failure to confirm association of vac A gene mosaicism with duodenal ulcer disease. Scand J Gastroenterol 33(2):132–136
Strobel S, Bereswill S, Balig P, Allgaier P, Sonntag HG, Kist M (1998) Identification and analysis of a new vacA genotype variant of Helicobacter pylori in different patient groups in Germany. J Clin Microbiol 36(5):1285–1289
Warburton VJ, Everett S, Mapstone NP, Axon AT, Hawkey P, Dixon MF (1998) Clinical and histological associations of cagA and vacA genotypes in Helicobacter pylori gastritis. J Clin Pathol 51(1):55–61
Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY (1998) Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter 3(4):241–253
Hennig EE, Trzeciak L, Regula J, Butruk E, Ostrowski J (1999) VacA genotyping directly from gastric biopsy specimens and estimation of mixed Helicobacter pylori infections in patients with duodenal ulcer and gastritis. Scand J Gastroenterol 34(8):743–749
Sadakane Y, Kusaba K, Nagasawa Z, Tanabe I, Kuroki S, Tadano J (1999) Prevalence and genetic diversity of cagD, cagE, and vacA in Helicobacter pylori strains isolated from Japanese patients. Scand J Gastroenterol 34(10):981–986
Audibert C, Janvier B, Grignon B, Salaun L, Burucoa C, Lecron JC, Fauchere JL (2000) Correlation between IL-8 induction, cagA status and vacA genotypes in 153 French Helicobacter pylori isolates. Res Microbiol 151(3):191–200
De Gusmao VR, Nogueira Mendes E, De Magalhaes Queiroz DM, Aguiar Rocha G, Camargos Rocha AM, Ramadan Ashour AA, Teles Carvalho AS (2000) vacA genotypes in Helicobacter pylori strains isolated from children with and without duodenal ulcer in Brazil. J Clin Microbiol 38(8):2853–2857
Figueiredo C, Van Doorn LJ, Nogueira C, Soares JM, Pinho C, Figueira P, Quint WG, Carneiro F (2001) Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scand J Gastroenterol 36(2):128–135
Miehlke S, Yu J, Schuppler M, Frings C, Kirsch C, Negraszus N, Morgner A, Stolte M, Ehninger G, Bayerdorffer E (2001) Helicobacter pylori vacA, iceA, and cagA status and pattern of gastritis in patients with malignant and benign gastroduodenal disease. Am J Gastroenterol 96(4):1008–1013. doi:10.1111/j.1572-0241.2001.03685.x
Park SM, Park J, Kim JG, Yoo BC (2001) Relevance of vacA genotypes of Helicobacter pylori to cagA status and its clinical outcome. Korean J Intern Med 16(1):8–13
Wong BC, Yin Y, Berg DE, Xia HH, Zhang JZ, Wang WH, Wong WM, Huang XR, Tang VS, Lam SK (2001) Distribution of distinct vacA, cagA and iceA alleles in Helicobacter pylori in Hong Kong. Helicobacter 6(4):317–324
Chattopadhyay S, Datta S, Chowdhury A, Chowdhury S, Mukhopadhyay AK, Rajendran K, Bhattacharya SK, Berg DE, Nair GB (2002) Virulence genes in Helicobacter pylori strains from West Bengal residents with overt H. pylori-associated disease and healthy volunteers. J Clin Microbiol 40(7):2622–2625
Choe YH, Kim PS, Lee DH, Kim HK, Kim YS, Shin YW, Hwang TS, Kim HJ, Song SU, Choi MS (2002) Diverse vacA allelic types of Helicobacter pylori in Korea and clinical correlation. Yonsei Med J 43(3):351–356
Ashour AA, Magalhaes PP, Mendes EN, Collares GB, de Gusmao VR, Queiroz DM, Nogueira AM, Rocha GA, de Oliveira CA (2002) Distribution of vacA genotypes in Helicobacter pylori strains isolated from Brazilian adult patients with gastritis, duodenal ulcer or gastric carcinoma. FEMS Immunol Med Microbiol 33(3):173–178
Smith SI, Kirsch C, Oyedeji KS, Arigbabu AO, Coker AO, Bayerdoffer E, Miehlke S (2002) Prevalence of Helicobacter pylori vacA, cagA and iceA genotypes in Nigerian patients with duodenal ulcer disease. J Med Microbiol 51(10):851–854
Brito CA, Silva LM, Juca N, Leal NC, de Souza W, Queiroz D, Cordeiro F, Silva NL (2003) Prevalence of cagA and vacA genes in isolates from patients with Helicobacter pylori-associated gastroduodenal diseases in Recife, Pernambuco Brazil. Mem Inst Oswaldo Cruz 98(6):817–821
Leodolter A, Wolle K, Peitz U, Ebert M, Gunther T, Kahl S, Malfertheiner P (2003) Helicobacter pylori genotypes and expression of gastritis in erosive gastro-oesophageal reflux disease. Scand J Gastroenterol 38(5):498–502
Perng CL, Lin HJ, Sun IC, Tseng GY, Facg (2003) Helicobacter pylori cagA, iceA and vacA status in Taiwanese patients with peptic ulcer and gastritis. J Gastroenterol Hepatol 18(11):1244–1249
Qiao W, Hu JL, Xiao B, Wu KC, Peng DR, Atherton JC, Xue H (2003) cagA and vacA genotype of Helicobacter pylori associated with gastric diseases in Xi’an area. World J Gastroenterol 9(8):1762–1766
Ribeiro ML, Godoy AP, Benvengo YH, Mendonca S, Pedrazzoli J Jr (2003) Clinical relevance of the cagA, vacA and iceA genotypes of Helicobacter pylori in Brazilian clinical isolates. FEMS Immunol Med Microbiol 36(3):181–185
Yin Y, Zhang JZ, Wang ZY, Xia HX, Lin ZX (2003) Association between Helicobacter pylori virulence and duodenal ulcer disease in patients from Hong Kong in China. Zhonghua Liu Xing Bing Xue Za Zhi 24(2):123–126
Ando T, Tsuzuki T, Mizuno T, Minami M, Ina K, Kusugami K, Takamatsu J, Adachi K, El-Omar E, Ohta M, Goto H (2004) Characteristics of Helicobacter pylori-induced gastritis and the effect of H. pylori eradication in patients with chronic idiopathic thrombocytopenic purpura. Helicobacter 9(5):443–452. doi:10.1111/j.1083-4389.2004.00261.x
Lin HJ, Perng CL, Lo WC, Wu CW, Tseng GY, Li AF, Sun IC, Ou YH (2004) Helicobacter pylori cagA, iceA and vacA genotypes in patients with gastric cancer in Taiwan. World J Gastroenterol 10(17):2493–2497
Perng CL, Lin HJ, Lo WC, Tseng GY, Sun IC, Ou YH (2004) Genotypes of Helicobacter pylori in patients with peptic ulcer bleeding. World J Gastroenterol 10(4):602–605
Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E (2005) A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci 50(8):1517–1522
Siavoshi F, Malekzadeh R, Daneshmand M, Ashktorab H (2005) Helicobacter pylori endemic and gastric disease. Dig Dis Sci 50(11):2075–2080. doi:10.1007/s10620-005-3010-1
Erzin Y, Koksal V, Altun S, Dobrucali A, Aslan M, Erdamar S, Dirican A, Kocazeybek B (2006) Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2 genotypes and correlation with clinical outcome in Turkish patients with dyspepsia. Helicobacter 11(6):574–580. doi:10.1111/j.1523-5378.2006.00461.x
Bolek BK, Salih BA, Sander E (2007) Genotyping of Helicobacter pylori strains from gastric biopsies by multiplex polymerase chain reaction. How advantageous is it? Diagn Microbiol Infect Dis 58(1):67–70. doi:10.1016/j.diagmicrobio.2006.12.001
Caner V, Yilmaz M, Yonetci N, Zencir S, Karagenc N, Kaleli I, Bagci H (2007) H. pylori iceA alleles are disease-specific virulence factors. World J Gastroenterol 13(18):2581–2585
Linpisarn S, Suwan W, Lertprasertsuk N, Koosirirat C, Steger HF, Prommuangyong K, Phornphutkul K (2007) Helicobacter pylori cagA, vacA and iceA genotypes in northern Thai patients with gastric disease. Southeast Asian J Trop Med Public Health 38(2):356–362
Proenca Modena JL, Lopes Sales AI, Olszanski Acrani G, Russo R, Vilela Ribeiro MA, Fukuhara Y, da Silveira WD, Modena JL, de Oliveira RB, Brocchi M (2007) Association between Helicobacter pylori genotypes and gastric disorders in relation to the cag pathogenicity island. Diagn Microbiol Infect Dis 59(1):7–16. doi:10.1016/j.diagmicrobio.2007.03.019
Tiwari SK, Khan AA, Manoj G, Ahmed S, Abid Z, Habeeb A, Habibullah CM (2007) A simple multiplex PCR assay for diagnosing virulent Helicobacter pylori infection in human gastric biopsy specimens from subjects with gastric carcinoma and other gastro-duodenal diseases. J Appl Microbiol 103(6):2353–2360. doi:10.1111/j.1365-2672.2007.03478.x
Miciuleviciene J, Calkauskas H, Jonaitis L, Kiudelis G, Tamosiunas V, Praskevicius A, Kupcinskas L, Berg D (2008) Helicobacter pylori genotypes in Lithuanian patients with chronic gastritis and duodenal ulcer. Medicina 44(6):449–454
Zhang Z, Zheng Q, Chen X, Xiao S, Liu W, Lu H (2008) The Helicobacter pylori duodenal ulcer promoting gene, dupA in China. BMC Gastroenterol 8:49. doi:10.1186/1471-230X-8-49
Bindayna KM, Al Mahmeed A (2009) vacA genotypes in Helicobacter pylori strains isolated from patients with and without duodenal ulcer in Bahrain. Indian J Gastroenterol 28(5):175–179. doi:10.1007/s12664-009-0069-1
Nagiyev T, Yula E, Abayli B, Koksal F (2009) Prevalence and genotypes of Helicobacter pylori in gastric biopsy specimens from patients with gastroduodenal pathologies in the Cukurova Region of Turkey. J Clin Microbiol 47(12):4150–4153. doi:10.1128/JCM.00605-09
Salehi Z, Abadi AS, Ismail PB, Kqueen CY, Jelodar MH, Kamalidehghan B (2009) Evaluation of Helicobacter pylori vacA genotypes in Iranian patients with peptic ulcer disease. Dig Dis Sci 54(11):2399–2403. doi:10.1007/s10620-008-0633-z
Torres LE, Melian K, Moreno A, Alonso J, Sabatier CA, Hernandez M, Bermudez L, Rodriguez BL (2009) Prevalence of vacA, cagA and babA2 genes in Cuban Helicobacter pylori isolates. World J Gastroenterol 15(2):204–210
Yakoob J, Abid S, Abbas Z, Jafri W, Ahmad Z, Ahmed R, Islam M (2009) Distribution of Helicobacter pylori virulence markers in patients with gastroduodenal diseases in Pakistan. BMC Gastroenterol 9:87. doi:10.1186/1471-230X-9-87
Dixit P, Sharma AK, Sinha SK, Prasad KK, Singh K (2011) Prevalence of cagA and vacA genotypes of H. pylori as a putative virulence marker in dyspeptic patients in northern India. J Gastroenterol Hepatol 26:276
Khan A, Farooqui A, Raza Y, Rasheed F, Manzoor H, Akhtar SS, Quraishy MS, Rubino S, Kazmi SU, Paglietti B (2013) Prevalence, diversity and disease association of Helicobacter pylori in dyspeptic patients from Pakistan. J Infect Dev Ctries 7(3):220–228. doi:10.3855/jidc.2942
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed.1000097
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwel lP (2011)The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute.www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed Dec. 1 2011
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188 0197-2456(86)90046-2 [pii]
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Coppede F, Bosco P, Lorenzoni V, Migheli F, Barone C, Antonucci I, Stuppia L, Romano C, Migliore L (2013) The MTR 2756A>G polymorphism and maternal risk of birth of a child with Down syndrome: a case–control study and a meta-analysis. Mol Biol Rep. doi:10.1007/s11033-013-2810-1
Li YY, Wu XY, Xu J, Qian Y, Zhou CW, Wang B (2013) Apo A5–1131T/C, FgB -455G/A, -148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: a meta-analysis of 15,055 subjects. Mol Biol Rep 40(2):1997–2014. doi:10.1007/s11033-012-2257-9
Letley DP, Atherton JC (2000) Natural diversity in the N terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J Bacteriol 182(11):3278–3280
Atherton JC, Peek RM Jr, Tham KT, Cover TL, Blaser MJ (1997) Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112(1):92–99
Acknowledgments
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, BB., Li, Y., Liu, XQ. et al. Association between vacA genotypes and the risk of duodenal ulcer: a meta-analysis. Mol Biol Rep 41, 7241–7254 (2014). https://doi.org/10.1007/s11033-014-3610-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3610-y