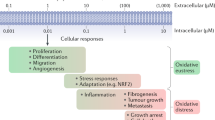
Abstract
In view of the critical role of redox system in numerous physiological and pathophysiological processes, it is important to clearly understand the family members and regulatory mechanism of redox system. In this work, we will systematically review the current data detailing the reactive oxygen species (ROS), enzymatic and non-enzymatic antioxidants and redox sensitive transcription factors and we give a brief description of redox-mediated epigenetic and post-translational regulation. We propose that the redox system functions as a “Redox Chain”, consisting of “ROS-generating Enzyme Chain”, “Combined Antioxidant Chain” and “Transcription Factor Chain”. We suggest that an individualized assessment of the redox status in the body should be conducted for the redox intervention of a patient. The strategy of intervention is to maintain redox homeostasis via either facilitation of ROS signaling or enhancement of antioxidant defense. These findings provide valuable new insights into redox system and open up new paths for the control of redox-related disorders








Similar content being viewed by others
Abbreviations
- 6PGD:
-
6-Phosphogluconate dehydrogenase
- 20-HETE:
-
20-Hydroxyeicosatetraenoic acid
- AA:
-
Arachidonic acid
- ADP:
-
Adenosine diphosphate
- AREs:
-
Antioxidant response elements
- ATP:
-
Adenosine triphosphate
- CAT:
-
Catalase
- CoQ:
-
Coenzyme Q
- COXs:
-
Cyclooxygenases
- CYPs:
-
Cytochrome P450 enzymes
- Drp1:
-
Dynamin-related protein
- EETs:
-
Epoxyeicosatrienoic acids
- EGR2:
-
Early growth response 2
- ER:
-
Endoplasmic reticulum
- ETC:
-
Electron transport cascade
- ETF-QO:
-
Electron transfer flavoprotein: ubiquinol oxidoreductase
- FATP-1:
-
Fatty acid transporter-1
- Fis1:
-
Fission 1 homologue protein
- FoxO:
-
Forkhead box O
- G6PDH:
-
Glucose-6-phosphate dehydrogenase
- GCL:
-
Glutamate–cysteine ligase
- GCLc:
-
Catalytic or heavy subunit
- GCLm:
-
Modulatory or light subunit
- GGT:
-
Γ-Glutamyl transpeptidase
- GPD2:
-
FAD-linked glycerol-3-phosphate dehydrogenase
- GPx:
-
GSH peroxidases
- GR:
-
GSH reductase
- Grxs:
-
Glutaredoxins
- GS:
-
Glutathione synthase
- GSH:
-
Glutathione
- GSSG:
-
Oxidized form of GSH
- GSSR:
-
Glutathionylated-cysteine derivative
- GSTs:
-
Glutathione-S-transferases
- HO:
-
Heme oxygenase
- Keap1:
-
Kelch-like ECH-associated protein 1
- KGDH:
-
Α-ketoglutarate dehydrogenase
- LA:
-
Lipoic acid
- LOX:
-
Lipoxygenases
- MAO:
-
Monoamine oxidase
- MAPKs:
-
Mitogen-activated protein kinase
- Mfn2:
-
Mitofusin 2
- MMO:
-
Microsomal monooxygenase
- MO:
-
Monooxygenase
- MTs:
-
Metallothioneins
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor kappa-light-cascade-enhancer of activated B cells
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthases
- NOXs:
-
NADPH oxidases
- NQO1:
-
NAD(P)H:quinone oxidoreductase 1
- Nrf2:
-
NFE2-related factor 2
- OPA1:
-
Optic atrophy-1
- OXPHOS:
-
Oxidative phosphorylation
- Ox-PTMs:
-
Oxidative post-translational modifications
- PGC-1α:
-
Peroxisome proliferatoractivated receptor-gamma coactivator-1α
- PGF2α :
-
Prostaglandin F2α
- PGs:
-
Prostaglandins
- PGE2 :
-
Prostaglandin E2
- PIG1-13:
-
P53-inducible genes 1-13
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- Prxs:
-
Peroxiredoxins
- PTMs:
-
Post-translational modifications
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SeP:
-
Selenoprotein P
- SHC1:
-
SHC-transforming protein 1
- Shh:
-
Sonic hedgehog
- SIRT:
-
Sirtuin
- SOD:
-
Superoxide dismutase
- SSAO:
-
Semicarbazide-sensitive amine oxidase
- TCA:
-
Tricarboxylic acid cycle
- TG:
-
Triglycerides
- TNF-α:
-
Tumor necrosis factor-α
- TrxR:
-
Thioredoxin reductase
- Trxs:
-
Thioredoxins
- TXNIP:
-
Thioredoxin interacting protein
- UCPs:
-
Uncoupling proteins
- UPR:
-
Uncoupling protein reaction
- VC :
-
Vitamin C
- VE :
-
Vitamin E
- XDH:
-
Xanthine dehydrogenase
- XO:
-
Xanthine oxidase
- XOR:
-
Xanthine oxidoreductase
References
Fenton HJH (1894) LXXIII-oxidation of tartaric acid in presence of iron. J Chem Soc Trans 65:899–910
May JM, de Haen C (1979) The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem 254:9017–9021
Lawrence JC, Larner J Jr (1978) J. Activation of glycogen synthase in rat adipocytes by insulin and glucose involves increased glucose transport and phosphorylation. J Biol Chem 253:2104–2113
Mukherjee SP, Lane RH, Lynn WS (1978) Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol 27:2589–2594
Wang X, Tao L, Hai CX (2012) Redox-regulating role of insulin: the essence of insulin effect. Mol Cell Endocrinol 349:111–127
Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C et al (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350(6266):1391–1396
Watson JD (2014) Type 2 diabetes as a redox disease. Lancet 383:841–843
Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468:1115–1118
Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468:696–700
Sun HJ, Zhao MX, Liu TY, Ren XS, Chen Q, Li YH, Kang YM, Zhu GQ (2016) Salusin-beta induces foam cell formation and monocyte adhesion in human vascular smooth muscle cells via miR155/NOX2/NFkappaB pathway. Sci Rep 6:23596
Wang X, Liu JZ, Hu JX, Wu H, Li YL, Chen HL, Bai H, Hai CX (2011) ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radical Bio Med 51:539–551
Rudzka DA, Cameron JM, Olson MF (2015) Reactive oxygen species and hydrogen peroxide generation in cell migration. Commun Integr Biol 8:e1074360
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520:57–62
Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF et al (2011) Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475:231–234
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H (2005) Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 7:1021–1031
Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ (2004) The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24:1844–1854
Shadel GS, Horvath TL (2015) Mitochondrial ROS signaling in organismal homeostasis. Cell 163:560–569
Yee C, Yang W, Hekimi S (2014) The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157:897–909
Gore A, Muralidhar M, Espey MG, Degenhardt K, Mantell LL (2010) Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol 7:239–254
Gorlach A, Dimova EY, Petry A, Martinez-Ruiz A, Hernansanz-Agustin P, Rolo AP, Palmeira CM, Kietzmann T (2015) Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol 6:372–385
Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S (2013) The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 65:1174–1194
Coates TD (2014) Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic Biol Med 72:23–40
Galaris D, Pantopoulos K (2008) Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci 45:1–23
Soberman RJ (2003) The expanding network of redox signaling: new observations, complexities, and perspectives. J Clin Invest 111:571–574
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
Turrens JF, Alexandre A, Lehninger AL (1985) Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237:408–414
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790
Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T (2000) Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol 20:7311–7318
Murphy M (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15
Singh G (2014) Mitochondrial FAD-linked Glycerol-3-phosphate dehydrogenase: a target for cancer therapeutics. Pharmaceuticals (Basel) 7:192–206
Hail N Jr, Chen P, Kepa JJ, Bushman LR, Shearn C (2010) Dihydroorotate dehydrogenase is required for N-(4-hydroxyphenyl)retinamide-induced reactive oxygen species production and apoptosis. Free Radic Biol Med 49:109–116
Watmough NJ, Frerman FE (2010) The electron transfer flavoprotein: ubiquinone oxidoreductases. Biochim Biophys Acta Bioenerg 1797:1910–1916
Lenaz G (2012) Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol 942:93–136
Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M (2008) p66Shc-generated oxidative signal promotes fat accumulation. J Biol Chem 283:34283–34293
Mailloux RJ, Harper ME (2012) Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab 23:451–458
Davydov DR (2001) Microsomal monooxygenase in apoptosis: another target for cytochrome c signaling? Trends Biochem Sci 26:155–160
Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS (2011) Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14:537–544
Yadav A, Agarwal S, Tiwari SK, Chaturvedi RK (2014) Mitochondria: prospective targets for neuroprotection in Parkinson’s disease. Curr Pharm Des 20:5558–5573
Apostolova N, Victor VM (2015) Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid Redox Signal 22:686–729
Brandes RP, Weissmann N, Schroder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76C:208–226
Sirokmany G, Donko A, Geiszt M (2016) Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol Sci 37:318–327
Martyn KD, Frederick LM, Loehneysen KV, Dinauer MC, Knaus UG (2006) Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18:69–82
Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth J, Shah A, Morel F, Brandes R (2011) The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286:13304–13313
Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob HJ, Schiffers P, Ho H, Wingler K, Schmidt HH (2012) The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69:2327–2343
Robert AM, Robert L (2014) Xanthine oxido-reductase, free radicals and cardiovascular disease. A critical review. Pathol Oncol Res 20:1–10
Sanders SA, Eisenthal R, Harrison R (1997) NADH oxidase activity of human xanthine oxidoreductase–generation of superoxide anion. Eur J Biochem 245:541–548
Cantu-Medellin N, Kelley EE (2013) Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide 34:19–26
Boueiz A, Damarla M, Hassoun PM (2008) Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am J Physiol Lung Cell Mol Physiol 294:L830–L840
Moncada S, Palmer RMJ, Higgs EA (1991) Nitricoxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–142
Jay-Gerin JP, Ferradini C (2000) Are there protective enzymatic pathways to regulate high local nitric oxide (NO) concentrations in cells under stress conditions? Biochimie 82:161–166
Lundberg JO, Gladwin MT, Weitzberg E (2015) Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14:623–641
Chen W, Lin YC, Ma XY, Jiang ZY, Lan SP (2014) High concentrations of genistein exhibit pro-oxidant effects in primary muscle cells through mechanisms involving 5-lipoxygenase-mediated production of reactive oxygen species. Food Chem Toxicol 67:72–79
Ha YJ, Seul HJ, Lee JR (2011) Ligation of CD40 receptor in human B lymphocytes triggers the 5-lipoxygenase pathway to produce reactive oxygen species and activate p38 MAPK. Exp Mol Med 43:101–110
Chou DS, Hsiao G, Lai YA, Tsai YJ, Sheu JR (2009) Baicalein induces proliferation inhibition in B16F10 melanoma cells by generating reactive oxygen species via 12-lipoxygenase. Free Radic Biol Med 46:1197–1203
Svensson HA, Bengtsson T, Grenegard M, Lindstrom EG (2008) Platelets stimulate airway smooth muscle cell proliferation through mechanisms involving 5-lipoxygenase and reactive oxygen species. Platelets 19:528–536
Kim C, Kim JY, Kim JH (2008) Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep 41:555–559
Cho KJ, Seo JM, Kim JH (2011) Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cells 32:1–5
Othman A, Ahmad S, Megyerdi S, Mussell R, Choksi K, Maddipati KR, Elmarakby A, Rizk N, Al-Shabrawey M (2013) 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS ONE 8:e57254
Yun MR, Park HM, Seo KW, Lee SJ, Im DS, Kim CD (2010) 5-Lipoxygenase plays an essential role in 4-HNE-enhanced ROS production in murine macrophages via activation of NADPH oxidase. Free Radic Res 44:742–750
Czapski GA, Czubowicz K, Strosznajder RP (2012) Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol Rep 64:1179–1188
Cole BK, Kuhn NS, Green-Mitchell SM, Leone KA, Raab RM, Nadler JL, Chakrabarti SK (2012) 12/15-Lipoxygenase signaling in the endoplasmic reticulum stress response. Am J Physiol Endocrinol Metab 302:E654–E665
Sancho P, Martin-Sanz P, Fabregat I (2011) Reciprocal regulation of NADPH oxidases and the cyclooxygenase-2 pathway. Free Radic Biol Med 51:1789–1798
Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K (2001) Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. J Biol Chem 276:12076–12083
Kim EH, Na HK, Kim DH, Park SA, Kim HN, Song NY, Surh YJ (2008) 15-Deoxy-Delta 12,14-prostaglandin J2 induces COX-2 expression through Akt-driven AP-1 activation in human breast cancer cells: a potential role of ROS. Carcinogenesis 29:688–695
Cederbaum AI, Wu D, Mari M, Bai J (2001) CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med 31:1539–1543
Bansal S, Liu CP, Sepuri NB, Anandatheerthavarada HK, Selvaraj V, Hoek J, Milne GL, Guengerich FP, Avadhani NG (2010) Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress. J Biol Chem 285:24609–24619
Ward NC, Puddey IB, Hodgson JM, Beilin LJ, Croft KD (2005) Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med 38:1032–1036
Han Y, Zhao H, Tang H, Li X, Tan J, Zeng Q, Sun C (2013) 20-Hydroxyeicosatetraenoic acid mediates isolated heart ischemia/reperfusion injury by increasing NADPH oxidase-derived reactive oxygen species production. Circ J 77:1807–1816
Zeng Q, Han Y, Bao Y, Li W, Li X, Shen X, Wang X, Yao F, O’Rourke ST, Sun C (2010) 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol 299:H1109–H1117
Eid S, Maalouf R, Jaffa AA, Nassif J, Hamdy A, Rashid A, Ziyadeh FN, Eid AA (2013) 20-HETE and EETs in diabetic nephropathy: a novel mechanistic pathway. PLoS ONE 8:e70029
Tu BP, Weissman JS (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164:341–346
Bhandary B, Marahatta A, Kim HR, Chae HJ (2012) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 14:434–456
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21:396–413
Santos CX, Tanaka LY, Wosniak J, Laurindo FR (2009) Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11:2409–2427
Kim HR, Lee GH, Cho EY, Chae SW, Ahn T, Chae HJ (2009) Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J Cell Sci 122:1126–1133
Nieto N, Friedman SL, Cederbaum AI (2002) Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology 35:62–73
Zangar RC, Davydov DR, Verma S (2004) Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199:316–331
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21:396–413
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Govatati S, Malempati S, Saradamma B, Divyamaanasa D, Naidu BP, Bramhachari PV, Narayana N, Shivaji S, Bhanoori M, Tamanam RR et al (2016) Manganese-superoxide dismutase (Mn-SOD) overexpression is a common event in colorectal cancers with mitochondrial microsatellite instability. Tumour Biol. doi:10.1007/s13277-016-4918-0
Batinic-Haberle I, Tovmasyan A, Roberts ER, Vujaskovic Z, Leong KW, Spasojevic I (2014) SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal 20:2372–2415
Houldsworth A, Hodgkinson A, Shaw S, Millward A, Demaine AG (2015) Polymorphic differences in the SOD-2 gene may affect the pathogenesis of nephropathy in patients with diabetes and diabetic complications. Gene 569:41–45
Doddigarla Z, Parwez I, Ahmad J (2015) Correlation of serum chromium, zinc, magnesium and SOD levels with HbA1c in type 2 diabetes: a cross sectional analysis. Diabetes Metab Syndr. doi:10.1016/j.dsx.2015.10.008
Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106:13576–13581
Baumgart K, Simkova V, Wagner F, Weber S, Georgieff M, Radermacher P, Albuszies G, Barth E (2009) Effect of SOD-1 over-expression on myocardial function during resuscitated murine septic shock. Intensive Care Med 35:344–349
Thomas R, Sharifi N (2012) SOD mimetics: a novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer. Mol Cancer Ther 11:87–97
Batinic-Haberle I (2011) SOD enzymes and their mimics in cancer: pro vs anti-oxidative mode of action. Part I. Anticancer Agents Med Chem 11:172–174
Stancic A, Otasevic V, Jankovic A, Vucetic M, Ivanovic-Burmazovic I, Filipovic MR, Korac A, Markelic M, Velickovic K, Golic I et al (2013) Molecular basis of hippocampal energy metabolism in diabetic rats: the effects of SOD mimic. Brain Res Bull 99:27–33
Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z et al (2006) A novel SOD-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free Radic Biol Med 41:835–848
Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB (2015) Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med 87:84–97
Hebert-Schuster M, Fabre EE, Nivet-Antoine V (2012) Catalase polymorphisms and metabolic diseases. Curr Opin Clin Nutr Metab Care 15:397–402
Lu J, Holmgren A (2014) The thioredoxin antioxidant system. Free Radic Biol Med 66:75–87
Tavakoli S, Asmis R (2012) Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal 17:1785–1795
Lu SC (1830) Glutathione synthesis. Biochim Biophys Acta 2013:3143–3153
Forman HJ, Zhang H, Rinna A (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med 30:1–12
Hwang C, Sinskey AJ, Lodish HF (1992) Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257:1496–1502
Baudouin-Cornu P, Lagniel G, Kumar C, Huang ME, Labarre J (2012) Glutathione degradation is a key determinant of glutathione homeostasis. J Biol Chem 287:4552–4561
Toppo S, Vanin S, Bosello V, Tosatto SC (2008) Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid Redox Signal 10:1501–1514
Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD (1997) Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 272:16644–16651
Chu FF, Doroshow JH, Esworthy RS (1993) Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase. GSHPx-GI. J Biol Chem 268:2571–2576
Takahashi K, Avissar N, Whitin J, Cohen H (1987) Purification and characterization of human plasma glutathione peroxidase: a selenoglycoprotein distinct from the known cellular enzyme. Arch Biochem Biophys 256:677–686
Yoshimura S, Watanabe K, Suemizu H, Onozawa T, Mizoguchi J, Tsuda K, Hatta H, Moriuchi T (1991) Tissue specific expression of the plasma glutathione peroxidase gene in rat kidney. J Biochem 109:918–923
Schneider M, Forster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, Neumuller C, Deutsch MJ, Walch A, Hrabe DAM et al (2009) Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J 23:3233–3242
Imai H, Hakkaku N, Iwamoto R, Suzuki J, Suzuki T, Tajima Y, Konishi K, Minami S, Ichinose S, Ishizaka K et al (2009) Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem 284:32522–32532
Chabory E, Damon C, Lenoir A, Kauselmann G, Kern H, Zevnik B, Garrel C, Saez F, Cadet R, Henry-Berger J et al (2009) Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 119:2074–2085
Brigelius-Flohe R (2006) Glutathione peroxidases and redox-regulated transcription factors. Biol Chem 387:1329–1335
Nguyen VD, Saaranen MJ, Karala AR, Lappi AK, Wang L, Raykhel IB, Alanen HI, Salo KE, Wang CC, Ruddock LW (2011) Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J Mol Biol 406:503–515
Wei PC, Hsieh YH, Su MI, Jiang X, Hsu PH, Lo WT, Weng JY, Jeng YM, Wang JM, Chen PL et al (2012) Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol Cell 48:747–759
Maiorino M, Ursini F, Bosello V, Toppo S, Tosatto SC, Mauri P, Becker K, Roveri A, Bulato C, Benazzi L et al (2007) The thioredoxin specificity of Drosophila GPx: a paradigm for a peroxiredoxin-like mechanism of many glutathione peroxidases. J Mol Biol 365:1033–1046
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Chen YJ, Lu CT, Lee TY (2014) dbGSH: a database of S-glutathionylation. Bioinformatics 30:2386–2388
Aquilano K, Baldelli S, Cardaci S, Rotilio G, Ciriolo MR (2011) Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. J Cell Sci 124:1043–1054
Aquilano K, Baldelli S, Pagliei B, Cannata SM, Rotilio G, Ciriolo MR (2013) p53 orchestrates the PGC-1alpha-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid Redox Signal 18:386–399
Sengupta R, Holmgren A (2013) Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid Redox Signal 18:259–269
Funato Y, Miki H (2007) Nucleoredoxin, a novel thioredoxin family member involved in cell growth and differentiation. Antioxid Redox Signal 9:1035–1057
Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT (2006) The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem 281:21884–21891
Chutkow WA, Lee RT (2011) Thioredoxin regulates adipogenesis through thioredoxin-interacting protein (Txnip) protein stability. J Biol Chem 286:29139–29145
Rhee SG, Woo HA, Kil IS, Bae SH (2012) Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem 287:4403–4410
Tan SX, Greetham D, Raeth S, Grant CM, Dawes IW, Perrone GG (2010) The thioredoxin-thioredoxin reductase system can function in vivo as an alternative system to reduce oxidized glutathione in Saccharomyces cerevisiae. J Biol Chem 285:6118–6126
Du Y, Zhang H, Lu J, Holmgren A (2012) Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J Biol Chem 287:38210–38219
Johansson C, Lillig CH, Holmgren A (2004) Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem 279:7537–7543
Ren P, Ye H, Dai L, Liu M, Liu X, Chai Y, Shao Q, Li Y, Lei N, Peng B et al (2013) Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol Rep 30:2297–2303
Zhu H, Santo A, Li Y (2012) The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood) 237:143–149
Holmgren A (1979) Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem 254:3672–3678
Lillig CH, Berndt C, Vergnolle O, Lonn ME, Hudemann C, Bill E, Holmgren A (2005) Characterization of human glutaredoxin 2 as iron-sulfur protein: a possible role as redox sensor. Proc Natl Acad Sci USA 102:8168–8173
Chertkov JL, Jiang S, Lutton JD, Levere RD, Abraham NG (1991) Hemin stimulation of hemopoiesis in murine long-term bone marrow culture. Exp Hematol 19:905–909
Kikuchi G, Yoshida T, Noguchi M (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338:558–567
Ollinger R, Yamashita K, Bilban M, Erat A, Kogler P, Thomas M, Csizmadia E, Usheva A, Margreiter R, Bach FH (2007) Bilirubin and biliverdin treatment of atherosclerotic diseases. Cell Cycle 6:39–43
Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N (2007) Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 27:1276–1282
Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG (2005) Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 289:H701–H707
Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S, Abraham NG (2007) Impact of silencing HO-2 on EC-SOD and the mitochondrial signaling pathway. J Cell Biochem 100:815–823
Abraham NG, Junge JM, Drummond GS (2016) Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends Pharmacol Sci 37:17–36
Dinkova-Kostova AT, Talalay P (2010) NAD(P)H:quinone accept or oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501:116–123
Park J, Rho HK, Kim KH, Choe SS, Lee YS, Kim JB (2005) Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol Cell Biol 25:5146–5157
Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF, Gesteland RF, Burk RF (2003) Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem 278:13640–13646
Kabuyama Y, Oshima K, Kitamura T, Homma M, Yamaki J, Munakata M, Homma Y (2007) Involvement of selenoprotein P in the regulation of redox balance and myofibroblast viability in idiopathic pulmonary fibrosis. Genes Cells 12:1235–1244
Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X, Hai C (2014) High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol Lett 224:16–23
Steinbrenner H, Bilgic E, Alili L, Sies H, Brenneisen P (2006) Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic Res 40:936–943
Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P (2006) Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med 40:1513–1523
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
Steven RD, Robert JC (2000) Metallothionein expression in animals: a physiological perspectuve on function. J Nutr 130:1085–1088
Futakawa N, Kondoh M, Ueda S, Higashimoto M, Takiguchi M, Suzuki S, Sato M (2006) Involvement of oxidative stress in the synthesis of metallothionein induced by mitochondrial inhibitors. Biol Pharm Bull 29:2016–2020
Ye B, Maret W, Vallee BL (2001) Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci USA 98:2317–2322
Moi P, Chan K, Asunis I, Cao A, Kan YW (1994) Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 91:9926–9930
Sekhar KR, Yan XX, Freeman ML (2002) Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene 21:6829–6834
Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24:7130–7139
Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29:493–502
Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140
Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M (2002) Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294:1–12
Sporn MB, Liby KT (2012) NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer 12:564–571
Kwak MK, Itoh K, Yamamoto M, Kensler TW (2002) Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22:2883–2892
Kay HY, Kim WD, Hwang SJ, Choi HS, Gilroy RK, Wan YJ, Kim SG (2011) Nrf2 inhibits LXRalpha-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid Redox Signal 15:2135–2146
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579:3029–3036
Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW et al (2005) Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA 102:4584–4589
Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT (2010) Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal 13:1713–1748
Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46:443–453
Tanito M, Agbaga MP, Anderson RE (2007) Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med 42:1838–1850
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I et al (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322
Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW (2003) Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278:8135–8145
Dong J, Sulik KK, Chen SY (2008) Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal 10:2023–2033
Favreau LV, Pickett CB (1995) The rat quinone reductase antioxidant response element. Identification of the nucleotide sequence required for basal and inducible activity and detection of antioxidant response element-binding proteins in hepatoma and non-hepatoma cell lines. J Biol Chem 270:24468–24474
Galloway DC, Blake DG, McLellan LI (1999) Regulation of gamma-glutamylcysteine synthetase regulatory subunit (GLCLR) gene expression: identification of the major transcriptional start site in HT29 cells. Biochim Biophys Acta 1446:47–56
Hintze KJ, Keck AS, Finley JW, Jeffery EH (2003) Induction of hepatic thioredoxin reductase activity by sulforaphane, both in Hepa1c1c7 cells and in male Fisher 344 rats. J Nutr Biochem 14:173–179
Ishii T, Itoh K, Sato H, Bannai S (1999) Oxidative stress-inducible proteins in macrophages. Free Radic Res 31:351–355
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Rushmore TH, Pickett CB (1990) Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem 265:14648–14653
Wang B, Williamson G (1994) Detection of a nuclear protein which binds specifically to the antioxidant responsive element (ARE) of the human NAD(P) H:quinone oxidoreductase gene. Biochim Biophys Acta 1219:645–652
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579:3029–3036
Zhang HS, Wang SQ (2007) Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells. Biochem Pharmacol 73:1358–1366
MacLeod AK, McMahon M, Plummer SM, Higgins LG, Penning TM, Igarashi K, Hayes JD (2009) Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis 30:1571–1580
Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT et al (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29:1235–1243
Jiang XY, Lu DB, Jiang YZ, Zhou LN, Cheng LQ, Chen B (2013) PGC-1alpha prevents apoptosis in adipose-derived stem cells by reducing reactive oxygen species production in a diabetic microenvironment. Diabetes Res Clin Pract 100:368–375
Austin S, Klimcakova E, St-Pierre J (2011) Impact of PGC-1alpha on the topology and rate of superoxide production by the mitochondrial electron transport chain. Free Radic Biol Med 51:2243–2248
Huang PI, Chou YC, Chang YL, Chien Y, Chen KH, Song WS, Peng CH, Chang CH, Lee SD, Lu KH et al (2011) Enhanced differentiation of three-gene-reprogrammed induced pluripotent stem cells into adipocytes via adenoviral-mediated PGC-1alpha overexpression. Int J Mol Sci 12:7554–7568
Huang PI, Chen YC, Chen LH, Juan CC, Ku HH, Wang ST, Chiou SH, Chiou GY, Chi CW, Hsu CC et al (2011) PGC-1alpha mediates differentiation of mesenchymal stem cells to brown adipose cells. J ATHEROSCLER THROMB 18:966–980
Baldelli S, Aquilano K, Ciriolo MR (2014) PGC-1alpha buffers ROS-mediated removal of mitochondria during myogenesis. Cell Death Dis 5:e1515
Yu W, Cao D, Zhou H, Hu Y, Guo T (2015) PGC-1alpha is responsible for survival of multiple myeloma cells under hyperglycemia and chemotherapy. Oncol Rep 33:2086–2092
Cao D, Jin L, Zhou H, Yu W, Hu Y, Guo T (2015) Inhibition of PGC-1alpha after chemotherapy-mediated insult confines multiple myeloma cell survival by affecting ROS accumulation. Oncol Rep 33:899–904
Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M (2005) PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 66:562–573
Sanchez-Ramos C, Tierrez A, Fabregat-Andres O, Wild B, Sanchez-Cabo F, Arduini A, Dopazo A, Monsalve M (2011) PGC-1alpha regulates translocated in liposarcoma activity: role in oxidative stress gene expression. Antioxid Redox Signal 15:325–337
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Geng T, Li P, Yin X, Yan Z (2011) PGC-1alpha promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am J Pathol 178:1738–1748
Hu L, Zhou L, Wu X, Liu C, Fan Y, Li Q (2014) Hypoxic preconditioning protects cardiomyocytes against hypoxia/reoxygenation injury through AMPK/eNOS/PGC-1alpha signaling pathway. Int J Clin Exp Pathol 7:7378–7388
Davies NA, Watkeys L, Butcher L, Potter S, Hughes MG, Moir H, Morris K, Thomas AW, Webb R (2015) The contributions of oxidative stress, oxidised lipoproteins and AMPK towards exercise-associated PPARgamma signalling within human monocytic cells. Free Radic Res 49:45–56
Irrcher I, Ljubicic V, Hood DA (2009) Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296:C116–C123
Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M (2006) Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J 20:1889–1891
Budanov AV (2014) The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell Biochem 85:337–358
Maillet A, Pervaiz S (2012) Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxid Redox Signal 16:1285–1294
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389:300–305
Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM (2005) The antioxidant function of the p53 tumor suppressor. Nat Med 11:1306–1313
Italiano D, Lena AM, Melino G, Candi E (2012) Identification of NCF2/p67phox as a novel p53 target gene. Cell Cycle 11:4589–4596
Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, Ichimiya M, Sengupta S, Mechanic L, Okamura S et al (2004) p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res 64:2350–2356
Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T (2004) Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119:847–860
Brynczka C, Labhart P, Merrick BA (2007) NGF-mediated transcriptional targets of p53 in PC12 neuronal differentiation. BMC Genom 8:139
Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM (2004) Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304:596–600
Kramer HF, Goodyear LJ (1985) Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol 2007(103):388–395
Kabe Y, Ando K, Hirao S, Yoshida M, Handa H (2005) Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 7:395–403
Zhang X, Liu J, Pang X, Zhao J, Wang S, Wu D (2014) Aldosterone induces C-reactive protein expression via MR-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Mol Cell Endocrinol 395:61–68
Jin X, Song L, Liu X, Chen M, Li Z, Cheng L, Ren H (2014) Protective efficacy of vitamins C and E on p, p’-DDT-induced cytotoxicity via the ROS-mediated mitochondrial pathway and NF-kappaB/FasL pathway. PLoS One 9:e113257
Kim SU, Park YH, Min JS, Sun HN, Han YH, Hua JM, Lee TH, Lee SR, Chang KT, Kang SW et al (2013) Peroxiredoxin I is a ROS/p38 MAPK-dependent inducible antioxidant that regulates NF-kappaB-mediated iNOS induction and microglial activation. J Neuroimmunol 259:26–36
Anrather J, Racchumi G, Iadecola C (2006) NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281:5657–5667
Pande D, Karki K, Negi R, Khanna S, Khanna RS, Khanna HD (2013) NF-kappaB p65 subunit DNA-binding activity: association with depleted antioxidant levels in breast carcinoma patients. Cell Biochem Biophys 67:1275–1281
Allen RG, Tresini M (2000) Oxidative stress and gene regulation. Free Radic Biol Med 28:463–499
Allan ME, Storey KB (2012) Expression of NF-kappaB and downstream antioxidant genes in skeletal muscle of hibernating ground squirrels, spermophilus tridecemlineatus. Cell Biochem Funct 30:166–174
Kaur P, Kaur G, Bansal MP (2006) Tertiary-butyl hydroperoxide induced oxidative stress and male reproductive activity in mice: role of transcription factor NF-kappaB and testicular antioxidant enzymes. Reprod Toxicol 22:479–484
Katoh M, Katoh M (2004) Human FOX gene family (review). Int J Oncol 25:1495–1500
de Keizer PL, Burgering BM, Dansen TB (2011) Forkhead box o as a sensor, mediator, and regulator of redox signaling. Antioxid Redox Signal 14:1093–1106
Calnan DR, Brunet A (2008) The FoxO code. ONCOGENE 27:2276–2288
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC et al (2008) ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 10:138–148
Honda Y, Honda S (1999) The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J 13:1385–1393
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321
Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424:277–283
Chiribau CB, Cheng L, Cucoranu IC, Yu YS, Clempus RE, Sorescu D (2008) FOXO3A regulates peroxiredoxin III expression in human cardiac fibroblasts. J Biol Chem 283:8211–8217
Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK (2014) FoxO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol 66:3349–3358
Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC, Liu GS (2013) Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22:878–888
Cherry AD, Suliman HB, Bartz RR, Piantadosi CA (2014) Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J Biol Chem 289:41–52
Sen N, Satija YK, Das S (2011) PGC-1α, a Key Modulator of p53, promotes cell survival upon metabolic stress. Mol Cell 44:621–634
Athale J, Ulrich A, MacGarvey NC, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA (2012) Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med 53:1584–1594
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD (2009) Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 34:663–673
Kalo E, Kogan-Sakin I, Solomon H, Bar-Nathan E, Shay M, Shetzer Y, Dekel E, Goldfinger N, Buganim Y, Stambolsky P et al (2012) Mutant p53R273H attenuates the expression of phase 2 detoxifying enzymes and promotes the survival of cells with high levels of reactive oxygen species. J Cell Sci 125:5578–5586
Kim DH, Song NY, Kim EH, Na HK, Joe Y, Chung HT, Surh YJ (2014) 15-Deoxy-delta 12,14-prostaglandin J(2) induces p53 expression through Nrf2-mediated upregulation of heme oxygenase-1 in human breast cancer cells. Free Radic Res 48:1018–1027
Asher G, Lotem J, Cohen B, Sachs L, Shaul Y (2001) Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc Natl Acad Sci USA 98:1188–1193
You A, Nam CW, Wakabayashi N, Yamamoto M, Kensler TW, Kwak MK (2011) Transcription factor Nrf2 maintains the basal expression of Mdm2: an implication of the regulation of p53 signaling by Nrf2. Arch Biochem Biophys 507:356–364
Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, Cimino F (2006) p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 281:39776–39784
Chen W, Jiang T, Wang H, Tao S, Lau A, Fang D, Zhang DD (2012) Does Nrf2 contribute to p53-mediated control of cell survival and death? Antioxid Redox Signal 17:1670–1675
George LE, Lokhandwala MF, Asghar M (2012) Novel role of NF-kappaB-p65 in antioxidant homeostasis in human kidney-2 cells. Am J Physiol Renal Physiol 302:F1440–F1446
Liu L, Shang Y, Li M, Han X, Wang J, Wang J (2015) Curcumin ameliorates asthmatic airway inflammation by activating Nrf2/HO-1 signaling pathway. Clin Exp Pharmacol Physiol 42(5):520–529
Cuadrado A, Martin-Moldes Z, Ye J, Lastres-Becker I (2014) Transcription factors NRF2 and NF-kappaB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem 289:15244–15258
Storci G, Sansone P, Mari S, D’Uva G, Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D, Chieco P et al (2010) TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol 225:682–691
Trebec-Reynolds DP, Voronov I, Heersche JN, Manolson MF (2010) VEGF-A expression in osteoclasts is regulated by NF-kappaB induction of HIF-1alpha. J Cell Biochem 110:343–351
Sun L, Zang WJ, Wang H, Zhao M, Yu XJ, He X, Miao Y, Zhou J (2014) Acetylcholine promotes ROS detoxification against hypoxia/reoxygenation-induced oxidative stress through FoxO3a/PGC-1alpha dependent superoxide dismutase. Cell Physiol Biochem 34:1614–1625
Yu DA, Yoon J, Ko YS, Park J, Kim SY, Kim MA, Kim JH, Jung J, Cheon Y, Lee HS et al (2014) Forkhead transcription factor FOXO1 inhibits nuclear factor-kappaB in gastric cancer. APMIS 122:848–855
Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA, Liu ZP (2009) FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology 137:1403–1414
Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K (2008) Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci 65:1049–1058
Kim DH, Kim JY, Yu BP, Chung HY (2008) The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology 9:33–47
Li Z, Zhang H, Chen Y, Fan L, Fang J (2012) Forkhead transcription factor FOXO3a protein activates nuclear factor kappaB through B-cell lymphoma/leukemia 10 (BCL10) protein and promotes tumor cell survival in serum deprivation. J Biol Chem 287:17737–17745
Huang J, Yue S, Ke B, Zhu J, Shen XD, Zhai Y, Yamamoto M, Busuttil RW, Kupiec-Weglinski JW (2014) Nuclear factor erythroid 2-related factor 2 regulates toll-like receptor 4 innate responses in mouse liver ischemia-reperfusion injury through Akt-forkhead box protein O1 signaling network. Transplantation 98:721–728
Zhang C, Shu L, Kong AT (2015) MicroRNAs: new players in cancer prevention targeting Nrf2, oxidative stress and inflammatory pathways. Curr Pharmacol Rep 1:21–30
Peng DF, Razvi M, Chen H, Washington K, Roessner A, Schneider-Stock R, El-Rifai W (2009) DNA hypermethylation regulates the expression of members of the Mu-class glutathione S-transferases and glutathione peroxidases in Barrett’s adenocarcinoma. Gut 58:5–15
Ren RJ, Dammer EB, Wang G, Seyfried NT, Levey AI (2014) Proteomics of protein post-translational modifications implicated in neurodegeneration. Transl Neurodegener 3:23
Victorino VJ, Mencalha AL, Panis C. Post-translational modifications disclose a dual role for redox stress in cardiovascular pathophysiology. LIFE SCI 2014
Waszczak C, Akter S, Jacques S, Huang J, Messens J, Van Breusegem F (2015) Oxidative post-translational modifications of cysteine residues in plant signal transduction. J Exp Bot 66(10):2923–2934
Tseng AH, Wu LH, Shieh SS, Wang DL (2014) SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J 464:157–168
Liu YB, Gao X, Deeb D, Arbab AS, Gautam SC (2013) Pristimerin induces apoptosis in prostate cancer cells by down-regulating Bcl-2 through ROS-dependent ubiquitin-proteasomal degradation pathway. J Carcinog Mutagen 6:5
Hori YS, Kuno A, Hosoda R, Horio Y (2013) Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One 8:e73875
Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, Liu P, Huang H (2013) Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med 65:528–540
Chen IC, Chiang WF, Liu SY, Chen PF, Chiang HC (2013) Role of SIRT3 in the regulation of redox balance during oral carcinogenesis. Mol Cancer 12:68
Tseng AH, Shieh SS, Wang DL (2013) SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 63:222–234
Chen HZ, Wan YZ, Liu DP (2013) Cross-talk between SIRT1 and p66Shc in vascular diseases. Trends Cardiovasc Med 23:237–241
Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, Perez-Vizcaino F, Jimenez R, Duarte J (2013) SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85:1288–1296
Kowalik-Jankowska T, Rajewska A, Jankowska E, Wisniewska K, Grzonka Z (2006) Products of Cu(II)-catalyzed oxidation of the N-terminal fragments of alpha-synuclein in the presence of hydrogen peroxide. J Inorg Biochem 100:1623–1631
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21:396–413
Avogaro A, de Kreutzenberg SV, Fadini GP (2008) Oxidative stress and vascular disease in diabetes: is the dichotomization of insulin signaling still valid? Free Radic Biol Med 44:1209–1215
Maiese K, Morhan SD, Chong ZZ (2007) Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res 4:63–71
Frank GD, Eguchi S, Motley ED (2005) The role of reactive oxygen species in insulin signaling in the vasculature. Antioxid Redox Signal 7:1053–1061
Wang X, Hai CX (2011) ROS acts as a double-edged sword in the pathogenesis of type 2 diabetes mellitus: is Nrf2 a potential target for the treatment? Mini Rev Med Chem 11:1082–1092
Pessler-Cohen D, Pekala PH, Kovsan J, Bloch-Damti A, Rudich A, Bashan N (2006) GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP alpha and delta isoforms in 3T3-L1 adipocytes. Arch Physiol Biochem 112:3–12
Lee H, Lee YJ, Choi H, Ko EH, Kim JW (2009) Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284:10601–10609
Doggrell SA (2004) Alpha-lipoic acid, an anti-obesity agent? Exp Opin Investig Drugs 13:1641–1643
Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS et al (2004) Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10:727–733
Lee OH, Seo DH, Park CS, Kim YC (2010) Puerarin enhances adipocyte differentiation, adiponectin expression, and antioxidant response in 3T3-L1 cells. BioFactors 36:459–467
Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF et al (2015) Corrigendum: selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 526:596
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO (2014) Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6:215r–221r
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 31400724) and Natural Science Foundation of Shaanxi Province (2014JQ4135).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Hai, C. Novel insights into redox system and the mechanism of redox regulation. Mol Biol Rep 43, 607–628 (2016). https://doi.org/10.1007/s11033-016-4022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4022-y