Abstract
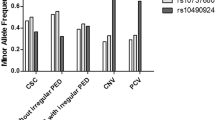
Glyoxalase 1 (GLO1) is a ubiquitous cellular enzyme involved in detoxification of methylglyoxal (MGO), a cytotoxic byproduct of glycolysis, whose excess can cause oxidative stress. In retinitis pigmentosa (RP), the prevalent cause of blindness just during working life in the industrialized countries, oxidative stress represents one of the possible mechanisms leading to death of cones following that of rods in the retina. To date, the causes of secondary death of cones remain unclear and among proposed mechanisms are: the deprivation of trophic factors normally produced by healthy rods, a compromised uptake of nutrients to cones due to irreversible destruction of RPE-cone outer segment, microglial activation and following release of pro-inflammatory cytokines and rod-derived toxins. In present paper, role of oxidative stress due to an excess of MGO was evaluated. In particular, we wanted to determine whether single nucleotide polymorphisms (SNPs) in GLO1 influence enzyme activity, contributing to cone death in advanced RP. 120 healthy controls and 80 RP patients from Sicilian population were genotyped for three GLO1 common SNPs, rs1130534 (c.372A>T, p.G124G), rs2736654 (c.A332C, p.E111A) and rs1049346 (c.-7C>T, 5′-UTR). While c.A332C polymorphism was not associated with RP, c.372A>T showed an allelic association (T372 allele frequency = 70% vs 60% in controls, p = 0.0071). Conversely, c.-7C>T showed both genotypic (χ2 = 68.0952; p = 1.634e−15) and allelic associations (χ2 = 51.7094; p = 6.435e−13): mutated allele frequency was higher in controls than in patients, suggesting its possible protective role. RP susceptibility may be associated with two of the analyzed GLO1 polymorphisms (rs1130534 and rs1049346).

Similar content being viewed by others
References
Richard JP (1993) Mechanism for the formation of methylglyoxal from triosephosphates. Biochem Soc Trans 21:549–553. https://doi.org/10.1042/bst0210549
Lyles GA, Chalmers J (1992) The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem Pharmacol 43:1409–1414. https://doi.org/10.1016/0006-2952(92)90196-P
Casazza JP, Felver ME, Veech RL (1984) The metabolism of acetone in rat. J Biol Chem 259:231–236
Hopper DJ, Cooper RA (1971) The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett 13:213–216. https://doi.org/10.1016/0014-5793(71)80538-0
Gonzalez FJ (1988) The molecular biology of cytochrome P450s. Pharmacol Rev 40:243–288
Yu PH, Wright S, Fan EH, Lun ZR, Gubisne-Harberle D (2003) Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim Biophys Acta 1647:193–199. https://doi.org/10.1016/S1570-9639(03)00101-8
Chang T, Wu L (2006) Methylglyoxal, oxidative stress, and hypertension. Can J Physiol Pharmacol 84:1229–1238. https://doi.org/10.1139/y06-077
Desai K, Wu L (2007) Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Pat Cardiovasc Drug Discov 2:89–99. https://doi.org/10.2174/157489007780832498
Mostafa AA, Randell EW, Vasdev SC, Gill VD, Han Y, Gadag V, Raouf AA, El Said H (2007) Plasma protein advanced glycation end products, carboxymethyl cysteine, and carboxyethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Mol Cell Biochem 302:35–42. https://doi.org/10.1007/s11010-007-9422-9
Bierhaus A, Nawroth PP (2009) Multiplelevels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 52:2251–2263. https://doi.org/10.1007/s00125-009-1458-9
Franke S, Dawczynski J, Strobel J, Niwa T, Stahl P, Stein G (2003) Increased levels of advanced glycation end products in human cataractous lenses. J Cataract Refract Surg 29:998–1004. https://doi.org/10.1016/S0886-3350(02)01841-2
Hong SB, Lee KW, Handa JT, Joo CK (2000) Effect of advanced glycation end products on lens epithelial cells in vitro. Biochem Biophys Res Commun 275:53–59. https://doi.org/10.1006/bbrc.2000.3245
Amicarelli F, Colafarina S, Cattani F, Cimini A, Di Ilio C, Ceru MP, Miranda P (2003) Scavenging system efficiency is crucial for cell resistance to ROS-mediated methylglyoxal injury. Free Radic Biol Med 35:856–871. https://doi.org/10.1016/S0891-5849(03)00438-6
Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S, Kasuga M (2005) Methylglyoxal induces apoptosis through oxidative stress-mediated activation of p38 mitogen-activated protein kinase in rat Schwann cells. Ann N Y Acad Sci 1043:151–157. https://doi.org/10.1196/annals.1333.019
Akhand AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, Kurokawa K, Nakashima I (2001) Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic Biol Med 31:20–30. https://doi.org/10.1016/S0891-5849(01)00550-0
Huang WJ, Tung CW, Ho C, Yang JT, Chen ML, Chang PJ, Lee PH, Lin CL, Wang JY (2007) Ras activation modulates methylglyoxal-induced mesangial cell apoptosis through superoxide production. Ren Fail 29:911–921. https://doi.org/10.1080/08860220701573509
Kim J, Son JW, Lee JA, Oh YS, Shinn SH (2004) Methylglyoxal induces apoptosis mediated by reactive oxygen species in bovine retinal pericytes. J Korean Med Sci 19:95–100. https://doi.org/10.3346/jkms.2004.19.1.95
Kalapos MP (1999) Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol Lett 110:145–175. https://doi.org/10.1016/S0378-4274(99)00160-5
Kikuchi S, Shinpo K, Moriwaka F, Makita Z, Miyata T, Tashiro K (1999) Neurotoxicity of methylglyoxal and 3-deoxyglucosone on cultured cortical neurons: synergism between glycation and oxidative stress, possibly involved in neurodegenerative diseases. J Neurosci Res 57:280–289. https://doi.org/10.1002/(SICI)1097-4547(19990715)57:2%3C280::AID-JNR14%3E3.0.CO;2-U
Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208. https://doi.org/10.1126/science.6836290
Shinpo K, Kikuchi S, Sasaki H, Ogata A, Moriwaka F, Tashiro K (2000) Selective vulnerability of spinal motor neurons to reactive dicarbonyl compounds, intermediate products of glycation, in vitro: implication of inefficient glutathione system in spinal motor neurons. Brain Res 861:151–159. https://doi.org/10.1016/S0006-8993(00)02047-3
Masuda T, Shimazawa M, Hara H (2017) Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (edaravone). Oxid Med Cell Longev 2017:9208489. https://doi.org/10.1155/2017/9208489
Boughman AJ, Conneally PM, Nance WE (1980) Population genetic studies of retinitis pigmentosa. Am J Hum Genet 32:223–235
Carmody RJ, McGowan AJ, Cotter TG (1999) Reactive oxygen species as mediators of photoreceptor apoptosis in vitro. Exp Cell Res 248:520–530. https://doi.org/10.1006/excr.1998.4421
Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A, Miki K, Iwase T, Lu L, Campochiaro PA (2009) NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem 110:1028–1037. https://doi.org/10.1111/j.1471-4159.2009.06195.x
Campochiaro PA, Mir TA (2018) The mechanism of cone cell death in retinitis pigmentosa. Prog Retin Eye Res 62:24–37. https://doi.org/10.1016/j.preteyeres.2017.08.004
Wong F, Kwok SY (2016) The survival of cone photoreceptors in retinitis pigmentosa. JAMA Ophthalmol 134:249–250. https://doi.org/10.1001/jamaophthalmol.2015.5490
Narayan DS, Wood JPM, Chidlow G, Casson RJ (2016) A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol 94:748–754. https://doi.org/10.1111/aos.13141
Junaid MA, Kowal D, Barua M, Pullarkat PS, Sklower Brooks S, Pullarkat RK (2004) Proteomic studies identified a single nucleotide polymorphism in glyoxalase I as autism susceptibility factor. Am J Med Genet A131:11–17. https://doi.org/10.1002/ajmg.a.30349
Rehnstrom K, Ylisaukko-Oja T, Vanhala R, von Wendt L, Peltonen L, Hovatta I (2008) No association between common variants in glyoxalase 1 and autism spectrum disorders. Am J Med Genet B 147:124–127. https://doi.org/10.1002/ajmg.b.30582
Sidoti A, Antognelli C, Rinaldi C, D’Angelo R, Dattola V, Girlanda P, Talesa V, Amato A (2007) Glyoxalase I A111E, paraoxonase 1 Q192R and L55M polymorphisms: susceptibility factors of multiple sclerosis? Mult Scler J 13:446–453. https://doi.org/10.1177/13524585070130040201
Rinaldi C, Bramanti P, Famà A, Scimone C, Donato L, Antognelli C, Alafaci C, Tomasello F, D’Angelo R, Sidoti A (2015) Glyoxalase I A111e, paraoxonase 1 Q192r and L55m polymorphisms in Italian patients with sporadic cerebral cavernous malformations: a pilot study. J Biol Regul Homeost Agents 29:493–500
Wu JC, Li XH, Peng YD, Wang JB, Tang JF, Wang YF (2011) Association of two glyoxalase I gene polymorphisms with nephropathy and retinopathy in Type 2 diabetes. J Endocrinol Invest 34:e343–e348. https://doi.org/10.3275/7856
Tao H, Si L, Zhou X, Liu Z, Ma Z, Zhou H, Zhong W, Cui L, Zhang S, Li Y, Ma G, Zhao J, Huang W, Yao L, Xu Z, Zhao B, Li K (2016) Role of glyoxalase I gene polymorphisms in late-onset epilepsy and drug-resistant epilepsy. J Neurol Sci 363:200–206. https://doi.org/10.1016/j.jns.2016.01.052
Scimone C, Bramanti P, Ruggeri A, Donato L, Alafaci C, Crisafulli C, Mucciardi M, Rinaldi C, Sidoti A, D’Angelo R (2016) CCM3/SERPINI1 bidirectional promoter variants in patients with cerebral cavernous malformations: a molecular and functional study. BMC Med Genet 17:74. https://doi.org/10.1186/s12881-016-0332-0
Miles JSM (2001) Applying regression & correlation: a guide for students and researchers. Sage, London
Donato L, Bramanti P, Scimone C, Rinaldi C, D’Angelo R, Sidoti A (2018) miRNA expression profile of retinal pigment epithelial (RPE) cells under oxidative stress conditions. FEBS Open Bio 8:219–233. https://doi.org/10.1002/2211-5463.12360
Peculis R, Konrade I, Skapare E, Fridmanis D, Nikitina-Zake L, Lejnieks A, Pirags V, Dambrova M, Klovins J (2013) Identification of glyoxalase 1 polymorphisms associatedwith enzyme activity. Gene 515:140–143. https://doi.org/10.1016/j.gene.2012.11.009
D’Angelo R, Donato L, Venza I, Scimone C, Aragona P, Sidoti A (2017) Possible protective role of the ABCA4 gene c.1268A>G missense variant in Stargardt disease and syndromic retinitis pigmentosa in a Sicilian family: preliminary data. Int J Mol Med 39:1011–1020. https://doi.org/10.3892/ijmm.2017.2917
Bair WB 3rd, Cabello CM, Uchida K, Bause AS, Wondrak GT (2010) GLO1 overexpression in human malignant melanoma. Melanoma Res 20:85–96. https://doi.org/10.1097/CMR.0b013e3283364903
Internet resources section
SNPator Web Based Software, http://www.snpator.org (October 9, 2017)
Arlequin Software 3.5.2.2, http://cmpg.unibe.ch/software/arlequin35/ (October 9, 2017)
G*Power Software 3.1, http://www.gpower.hhu.de (October 9, 2017)
Haploview software, https://www.broadinstitute.org/haploview/haploview (October 9, 2017)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Informed consent
All subjects had given written informed consent prior to participation in the study.
Research involving human participants
This study was approved by the Ethics Committee of “Azienda Policlinico Universitario of Messina” and conformed to the tenets of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Donato, L., Scimone, C., Nicocia, G. et al. GLO1 gene polymorphisms and their association with retinitis pigmentosa: a case–control study in a Sicilian population. Mol Biol Rep 45, 1349–1355 (2018). https://doi.org/10.1007/s11033-018-4295-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4295-4