Abstract
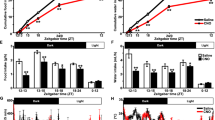
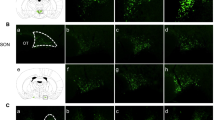
Arginine vasopressin (AVP) is known to a neuropeptide that plays important roles in water conservation, sodium homeostasis, and in the regulation of serum osmolality. Several studies have reported that the elevated AVP level is related with diabetes mellitus as an acute or chronic stressor using type 1 diabetes mellitus animal models. However, it is unclear as to how the immunoreactivity and protein level of AVP in the brain is regulated in animal models of type 2 diabetes mellitus. In the present study, Zucker diabetic fatty (ZDF) rats were employed as a type 2 diabetes mellitus model and were compared with Zucker lean control (ZLC) rats with respect to AVP protein expression. Furthermore, in order to verify the regulation of AVP expression before and after the onset of diabetes mellitus, pre-diabetic rats (4 week-old) and obese-diabetic rats (12 week-old) were used. Blood glucose levels and water consumption were also measured and the results showed significantly high in 12 week-old ZDF than any other groups. AVP expression levels in the paraventricular nucleus and supraoptic nucleus were found to be significantly higher in 12 week-old ZDF rats than in 12 week-old ZLC rats and than in 4 week-old rats by immunostaining and western blotting. Enhanced expression of AVP in these animals may be associated with type 2 diabetes mellitus.







Similar content being viewed by others
References
Zelena D, Filaretova L, Mergl Z, Barna I, Toth ZE, Makara GB (2006) Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am J Physiol Endocrinol Metab 290:243–250
Franco-Bourland RE (1998) Vasopressinergic, oxytocinergic, and somatostatinergic neuronal activity after adrenalectomy and immobilization stress. Neurochem Res 23:695–701
Medeiros Mdos S, Turner AJ (1996) Metabolism and function of neuropeptide Y. Neurochem Res 21:1125–1132
Antoni FA (1999) Vasopressinergic control pituitary adrenocorticotropin secretion comes of age. Clin Sci (Lond) 96:513–523
Scatt LV, Dinan TG (1998) Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci 62:1985–1998
Van Vugt DA, Lujan ME, Froats M, Krzemien A, Couceyro PR, Reid RL (2006) Effect of fasting on cocaine-amphetamine-regulated transcript, neuropeptide Y, and leptin receptor expression in the non-human primate hypothalamus. Neuroendocrinology 84:83–93
Morita M, Kita Y, Morikawa N, Iwami M, Notsu Y (2001) Expression of arginine vasopressin and vasopressin V1a receptor mRNA in diabetic (db/db) mice. Exp Clin Endocrinol Diabetes 109:261–266
Saravia FE, Gonzalez SL, Roig P, Alves V, Homo-Delarche F, De Nicola AF (2001) Diabetes increases the expression of hypothalamic neuropeptides in a spontaneous model of type I diabetes, the nonobese diabetic (NOD) mouse. Cell Mol Neurobiol 21:15–27
Hayashi M, Arima H, Goto M et al (2006) Vasopressin gene transcription increases in response to decrease in plasma volume, but not to increase in plasma osmolality, in chronically dehydrated rats. Am J Physiol Endocrinol Metab 290:E213–E217
Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M (1994) Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol 6:369–373
Neumann I, Landgraf R, Bauce L, Pittman QJ (1995) Osmotic responsiveness and cross talk involving oxytocin, but not vasopressin or amino acids, between the supraoptic nuclei in virgin and lactating rats. J Neurosci 15:3408–3417
Bankir L, Bardoux P, Ahloulay M (2001) Vasopressin and diabetes mellitus. Nephron 87:8–18
Luo Y, Kaur C, Ling EA (2002) Neuronal and glial response in the rat hypothalamus-neurohypophysis complex with streptozotocin-induced diabetes. Brain Res 925:42–54
Yibchok-anun S, Abu-Basha EA, Yao CY, Panichkriangkrai W, Hsu WH (2004) The role of arginine vasopressin in diabetes-associated increase in glucagons secretion. Regul Pept 122:157–162
Kim MJ, Hong SJ, Yang J, Kim HK (2007) Silkworm (Bombyx mori L.) reduces vasopressin expression in the hypothalamus of streptozotocin-induced diabetic mice. Neurol Res 29:S72–S77
Chua SC Jr, Chung WK, Wu-Peng XS et al (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271:994–996
Lugarini F, Hrupka BJ, Schwartz GJ, Plata-Salaman CR, Langhans W (2005) Acute and chronic administration of immunomodulator induces anorexia in Zucker rats. Physiol Behav 84:165–173
Peterson RG (2001) The zucker diabetic fatty (ZDF) rat. In: Sima AAF, Shafrir E (eds) Animal models of diabetes a primer. Harwood academic publishers, Amsterdam, pp 109–128
Harmons JS, Gleason CE, Tanaka Y, Poitout V, Robertson RP (2001) Antecedent hyperglycemia, not hyperlipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes 50:2481–2486
Hwang IK, Yi SS, Kim YN et al (2007) Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res doi. 10.1007/s11064-007-9940-8
Dheen ST, Tay SS, Wong WC (1994) Arginine vasopressin- and oxytocin-like immunoreactive neurons in the hypothalamic paraventricular and supraoptic nuclei of streptozotocin-induced diabetic rats. Arch Histol Cytol 57:461–472
Zelena D, Mergl Z, Makara GB (2006) The role of vasopressin in diabetes mellitus-induced hypothalamo-pituitary-adrenal axis activation: studies in Barttleboro rats. Brain Res Bull 69:48–56
Vokes TP, Aycinena PR, Rebertson GL (1987) Effect of insulin on osmoregulation of vasopressin. Am J Physiol 255:E538–E548
Chan O, Inouye K, Riddell MC, Vranic M, Matthews SG (2003) Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol 28:87–102
Anai H, Ueta Y, Serino R et al (1997) Upregulation of the expression of vasopressin gene in the paraventricular and supraoptic nuclei of the lithium-induced diabetes insipidus rat. Brain Res 772:161–166
Gillard ER, Coburn CG, de Leon A et al (2007) Vasopressin autoreceptors and nitric oxide-dependent glutamate release are required for somatodendritic vasopressin release from rat magnocellular neuroendocrine cells responding to osmotic stimuli. Endocrinology 148:479–489
Fujiwara Y, Hiroyama M, Sanbe A et al (2007) Insulin hypersensitivity in mice lacking the V1b vasopressin receptor. J Physiol doi:10.1113/jphysiol.2007.136481
Walsh CH, Baylis PH, Malins JM (1979) Plasma arginine vasopressin in diabetic ketoacidosis. Diabetologia 16:93–96
Zerbe RL, Vinicor F, Robertson GL (1979) Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes 28:503–508
Hems DA, Ma GY (1976) Resistance to hepatic action of vasopressin in genetically obese (ob/ob) mice. Biochem J 160:23–28
McKenna K, Morris AD, Ryan M et al (2000) Renal resistance to vasopressin in poorly controlled type 1 diabetes mellitus. Am J Physiol Endocrinol Metab 279:E155–E160
Agha A, Smith D, Finucane F et al (2004) Attenuation of vasopressin-induced antidiuresis in poorly controlled type 2 diabetes. Am J Physiol Endocrinol Metab 287:E1100–E1106
Waldhausl W, Bratusch-Marrain P, Gasic S, Korn A, Nowotny P (1982) Insulin production rate, hepatic insulin retention, and splanchnic carbohydrate metabolism after oral glucose ingestion in hyperinsulinemic type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 23:6–15
Aoyagi T, Birumachi J, Hiroyama M et al (2007) Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 148:2075–2084
Lee B, Yang C, Chen TH, al-Azawi N, Hsu WH (1995) Effect of AVP and oxytocin on insulin release: involvement of V1b receptor. Am J Physiol 269:E1095–E1100
Richardson SB, Laya T, VanOoy M (1995) Similarities between hamster pancreatic islet beta (HIT) cell vasopressin receptors and V1b receptors. J Endocrinol 147:59–65
Yibchok-anun S, Cheng H, Heine PA, Hsu WH (1999) Characterization of receptors mediating AVP- and OT- induced glucagons release from the rat pancreas. Am J Physiol 277:E56–E62
Yibchok-anun S, Hsu WH (1998) Effects of arginine vasopressin and oxytocin on glucagons release from clonal alpha-cell line In-R1-G9: involvement of V1b receptors. Life Sci 63:1871–1878
Folny V, Raufaste D, Likovic L et al (2003) Pancreatic vasopressin V1b receptors: characterization in In-R1-G9 cells and localization in human pancreas. Am J Physiol Endocrinol Metab 285:E566–E576
Acknowledgements
This work was supported by the Grants of MRC for Chronic Metabolic Syndrome from the Ministry of Health and Welfare in Korea to J. K. Seong and Research Institute for Veterinary Science, Seoul National University to Y. S. Yoon.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Special issue article in honor of George Fink.
Rights and permissions
About this article
Cite this article
Yi, S.S., Hwang, I.K., Kim, Y.N. et al. Enhanced Expressions of Arginine Vasopressin (Avp) in the Hypothalamic Paraventricular and Supraoptic Nuclei of Type 2 Diabetic Rats. Neurochem Res 33, 833–841 (2008). https://doi.org/10.1007/s11064-007-9519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9519-2