Abstract
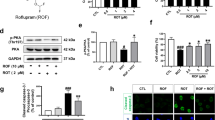
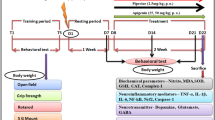
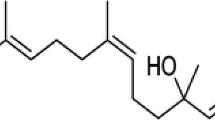
Pramipexole (PPX), a dopamine (DA) receptor D3 preferring agonist, has been used as monotherapy or adjunct therapy to treat Parkinson’s disease (PD) for many years. Several in vitro and in vivo studies in neurotoxin-induced DA neuron injury models have reported that PPX may possess neuroprotective properties. The present study is to evaluate the neuroprotection of PPX in a sustained DA neuron degeneration model of PD induced by ubiquitin–proteasome system (UPS) impairment. Adult C57BL/6 mice were treated with PPX (low dose 0.1 mg/kg or high dose 0.5 mg/kg, i.p, twice a day) started 7 days before, and continued after microinjection of proteasome inhibitor lactacystin in the medial forebrain bundle for a total 4 weeks. Animal behavior observation, and pathological and biochemical assays were conducted to determine the neuroprotective effects of PPX. We report here that PPX treatment significantly improves rotarod performance, attenuates DA neuron loss and striatal DA reduction, and alleviates proteasomal inhibition and microglial activation in the substantia nigra of lactacystin-lesioned mice. PPX can increase the levels of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor and induce an activation of autophagy. Furthermore, pretreatment with D3 receptor antagonist U99194 can significantly block the PPX-mediated neuroprotection. These results suggest that multiple molecular pathways may be attributed to the neuroprotective effects of PPX in the UPS impairment model of PD.








Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ALP:
-
Autophagy lysosome pathway
- BDNF:
-
Brain-derived neurotrophic factor
- DA:
-
Dopamine
- DAB:
-
3, 3-diaminobenzidine tetrachloride
- DOPAC:
-
4-dihydroxy-phenylacetic acid
- DPBS:
-
Dulbecco’s phosphate buffered saline
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GFAP:
-
Glial fibrillary acidic protein
- HD:
-
Huntington’s disease
- HVA:
-
Homovanillic acid
- LC3–II:
-
Light chain3–II
- MFB:
-
Medial forebrain bundle
- mTOR:
-
Mammalian target of rapamycin
- PBS:
-
Phosphate-buffered saline
- PD:
-
Parkinson’s disease
- ppx:
-
Pramipexole
- sn:
-
Substantia nigra
- TH:
-
Tyrosine hydroxylase
- UPS:
-
Ubiquitin proteasome system
References
Snyder H, Wolozin B (2004) Pathological proteins in Parkinson’s disease: focus on the proteasome. J Mol Neurosci 24:425–442
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Ann Rev Neurosci 28:57–87
Le W, Chen S, Jankovic J (2009) Etiopathogenesis of Parkinson disease: a new beginning? Neuroscientist 15:28–35
Barone P (2003) Clinical strategies to prevent and delay motor complications. Neurology 61:S12–S16
Jankovic J (2006) An update on the treatment of Parkinson’s disease. Mt Sinai J Med 73:682–689
Clarke CE, Guttman M (2002) Dopamine agonist monotherapy in Parkinson’s disease. Lancet 360:1767–1769
Shannon KM, Bennett JP Jr, Friedman JH (1997) Efficacy of pramipexole a novel dopamine agonist, in mild to moderate Parkinson’s disease. The pramipexole study group. Neurology 49:724–728
Albrecht S, Buerger E (2009) Potential neuroprotection mechanisms in PD: focus on dopamineagonist pramipexole. Curr Med Res Opin 25:2977–2987
Le WD, Jankovic J (2001) Are dopamine receptor agonists neuroprotective in Parkinson’s disease? Drugs Aging 18:389–396
Zou LL, Xu J, Jankovic J, He Y, Appel SH, Le W (2000) Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in C57BL/6 mice. Neurosci Lett 281:167–170
Cassarino DS, Fall CP, Smith TS, Bennett JP Jr (1998) Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. J Neurochem 71:295–301
Joyce JN, Millan MJ (2007) Dopamine D3 receptor agonists for protection and repair in Parkinson’s disease. Curr Opin Pharmacol 7:100–105
Olanow CW, Kordower JH (2009) Modeling Parkinson’s disease. Ann Neurol 66:432–435
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Höglinger GU, Oertel WH, Hirsch EC (2006) The rotenone model of parkinsonism–the five years inspection. J Neural Transm Suppl 70:269–272
Sun F, Kanthasamy A, Anantharam V, Kanthasamy AG (2007) Environmental neurotoxic chemicals-induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson’s disease. Pharmacol Ther 114:327–344
Cook C, Petrucelli L (2009) A critical evaluation of the ubiquitin-proteasome system in Parkinson’s disease. Biochem Biophys Acta 1792:664–675
Bennett EJ, Bence NF, Jayakumar R, Kopito RR (2005) Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell 17:351–365
McNaught KS, Olanow CW (2006) Protein aggregation in the pathogenesis of familial and sporadic Parkinson’s disease. Neurobiol Aging 27:530–545
Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, Gray T, Topham I, Fone K, Rezvani N, Mee M, Soane T, Layfield R, Sheppard PW, Ebendal T, Usoskin D, Lowe J, Mayer RJ (2008) Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci 28:8189–8198
Zhang X, Xie WJ, Qu S, Pan T, Wang X, Le W (2005) Neuroprotection by iron chelator against proteasome inhibitor-induced nigral degeneration. Biochem Biophys Res Commun 333:544–549
Zhu W, Xie WJ, Pan TH, Jankovic J, Li J, Youdim MB, Le W (2007) Prevention and restoration of lactacystin-induced nigrostriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J 21:3835–3844
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Martinez-Vicente M, Cuervo AM (2007) Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol 6:352–361
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884
Glaser JR, Glaser EM (2000) Stereology, morphometry, and mapping: the whole is greater than the sum of its parts. J Chem Neuroanat 20:115–126
Du F, Li R, Huang Y, Li X, Le W (2005) Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci 22:2422–2430
Zemke D, Azhar S, Majid A (2007) The mTOR pathway as a potential target for the development of therapies against neurological disease. Drug News Perspect 20:495–499
Pan TH, Kondo S, Zhu W, Xie W, Jankovic J, Le W (2008) Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis 32:16–25
Acknowledgments
This work is supported by Research Grant from Boehringer Ingelheim Pharma GmbH & Co. KG Research Grant, and Diana Helis Henry Medical Research Foundation We also thank the High Resolution Electron Microscopy Facility, University of Texas MD Anderson for their help in electronic microscope examination.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chao Li and Yuan Guo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, C., Guo, Y., Xie, W. et al. Neuroprotection of Pramipexole in UPS Impairment Induced Animal Model of Parkinson’s Disease. Neurochem Res 35, 1546–1556 (2010). https://doi.org/10.1007/s11064-010-0214-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0214-3