Abstract
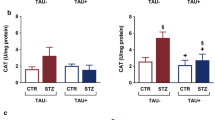
The mechanisms underlying diabetic encephalopathy, are only partially understood. In this study, we try to address the mechanisms of diabetes induced damage and whether docosahexaenoic acid (DHA) could attenuate the degenerative changes in diabetic hippocampus in a rodent model of diabetes. Diabetes was induced in rats by an intraperitoneal injection of streptozotocin. Animals were divided into the following experimental groups: control rats; control animals treated with DHA; untreated diabetic rats; diabetic rats treated with insulin; diabetic rats treated with DHA; diabetic rats treated with insulin and DHA. At the end of week 12, rats were killed and one of the hemispheres was cryosectioned and the other was dissected and hippocampi homogenized. The number of bromodeoxyuridine positive cells in the hippocampus of diabetic rats was decreased, and the latency time to find the platform in the Morris Water maze was significantly increased in the diabetic rats when compared to controls. No changes where observed in the expression of p21 in the hippocampus of control and diabetic rats. Biochemical markers of oxidative stress were altered in hippocampus of diabetic rats, and NFκB-positive cells were increased in the hippocampus of diabetic rats when compared to controls. Treatment with DHA, or the combination of DHA with insulin, significantly restored to control levels all the values mentioned above. Our findings confirm a pivotal role for oxidative stress as well as NF-κB, but not p21, in diabetes-induced hippocampal impairments. Administration of DHA as well as insulin prevented the changes induced by diabetes in hippocampus.









Similar content being viewed by others
References
Ryan CM, Williams TM, Finegold DN, Orchard TJ (1993) Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia 36:329–334
Flood JF, Mooradian AD, Morley JE (1990) Characteristics of learning and memory in streptozotocin-induced diabetic mice. Diabetes 39:1391–1398
Beauquis J, Saravia F, Coulaud J, Roig P, Dardenne M et al (2008) Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol 210:359–367
Jackson-Guilford J, Leander J, Nisenbaum L (2000) The effect of streptozotocin induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett 293:91–94
Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM (2008) Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand 117:205–210
Puchowicz MA, Xu K, Magnes D, Miller C, Lust WD, Kern TS, LaManna JC (2004) Comparison of glucose influx and blood flow in retina and brain of diabetic rats. J Cereb Blood Flow Metab 24:449–457
Shors TJ, Miesegaes G, Baylin A, Zhao M, Rydel TGE (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376
Makimattila S, Malmberg-Ceder K, Hakkinen AN, Vuori K, Salonen O, Summanen P, Yki-Jarvinen K, Kaste M, Heikkinen S, Lundbom M, Roine RO (2004) Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab 24:1393–1399
Kempermann G, Gage FH (1999) New nerve cells for the adult brain. Sci Am 280:48–53
Gould E, Deylin A, Tanapat T, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265
Herrera DG, Yagüe AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, García-Verdugo JM (2003) Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA 100:7919–7924
Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM et al (1996) Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes 45:1259–1266
Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW et al (1998) Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res 800:125–135
Yamada KA, Rensing N, Izumi Y, Erausquin GAD, Gazit V, Dorsey DA, Herrera DG (2004) The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Pediatr Res 55:372–379
Li Z, Zhang W, Grunberger G, Sima AF (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res 946:212–231
Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V (2008) p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci USA 105:1358–1363
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132:344–362
Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, Bours V (1997) Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem 378:1237–1245
Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG (2001) NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev 12:73–90
Seitz CS, Deng H, Hinata K, Lin Q, Khavari PA (2000) Nuclear factor kappaB subunits induce epithelial cell growth arrest. Cancer Res 60:4085–4092
Liu GH, Qu J, Shen X (2008) NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 1783:713–727
He C, Qu X, Cui L, Wang J, Kang JX (2009) Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci USA 106:11370–11375
Bazan NG (2005) Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol 15:159–166
Martin RE, Bazan NG (1992) Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem 59:318–325
Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG (2000) A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 42:174–181
Gamoh S, Hashimoto M, Hossain S, Masumura S (2001) Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol 28:266–270
Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N Jr (2007) An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot Essent Fatty Acids 77:269–277
Wang TM, Chen CJ, Lee TS, Chao HY, Wu WH, Hsieh SC, Sheu HH, Chiang AN (2011) Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. Nutr Biochem 22(2):187–194
Bazan NG (2006) Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 29:263–271
Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG (2004) Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA 101:8491–8496
Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG (2003) Novel docosanoids inhibit brain ischemia-reperfusion mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem 278:43807–43817
Richard MJ, Guiraud P, Meo J, Favier A (1992) High performance liquid chromatography separation of malondialdehyde thiobarbituric acid adduct in biological materials (plasma and human cell) using a commercially available reagent. J Chromatogr 577:9–18
Romero MJ, Bosch-Morell F, Romero B, Rodrigo JM, Serra MA, Romero FJ (1998) Serum malondialdehyde: possible use for the clinical management of chronic hepatitis C patients. Free Radical Biol Med 25:993–997
Lawrence RA, Parkhill LK, Burk RF (1978) Hepatic cytosolic non-selenium dependent glutathione peroxidase activity: its nature and the effect of selenium deficiency. J Nutr 108:981–987
Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW (1980) High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related disulfides. Anal Biochem 106:55–62
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Messier C (2005) Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 26:26–30
Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP (2008) Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11:309–317
O’Neill LA, Kaltschmidt C (1997) NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20:252–258
Mattson MP, Camandola S (2001) NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107:247–254
Tergaonkar V (2006) NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol 38:1647–1653
Grilli M, Memo M (1999) Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Div Differ 6:22–27
Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G (2003) Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci USA 100:9566–9571
Fujioka T, Fujioka A, Duman RS (2004) Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci 24:319–328
Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS (2002) Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci 22:9868–9876
Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS (2002) Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 22:3673–3682
Zou J, Crews F (2006) CREB and NF-κB transcription factors regulate densitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell Mol Neurobiol 26:4–6
Shenkar R, Yum HK, Arcaroli J, Kupfner J, Abraham E (2001) Interactions between CBP, NF-kappaB, and CREB in the lungs after hemorrhage and endotoxemia. Am J Physiol Lung Cell Mol Physiol 281:L418–L426
Wang W, Shinto L, Connor WE, Quinn JF (2008) Biomarkers in Alzheimer’s disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J Alzheimer’s Dis 13:31–38
Acknowledgments
This work was supported in part by Funds from Ministerio de Ciencia e Innovación (SAF 2010-21317) and Copernicus-Santander (PRCEU-UCH/COP(01)10/11) programme of the Universidad CEU—Cardenal Herrera.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez-Nölting, R., Arnal, E., Barcia, J.M. et al. Protection by DHA of Early Hippocampal Changes in Diabetes: Possible Role of CREB and NF-κB. Neurochem Res 37, 105–115 (2012). https://doi.org/10.1007/s11064-011-0588-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0588-x