Abstract
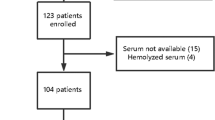
S100β and neuron-specific enolase (NSE) are brain injury biomarkers, mainly used in brain trauma, cerebral stroke and hypoxic ischemia encephalopathy. The aim of this study was to study the clinical significance of serum S100β and NSE in diagnosing sepsis-associated encephalopathy (SAE) and predicting its prognosis. This was a prospective and observational study. Clinical data of septic patients were collected within 24 h after ICU admission from May 2012 to April 2013. We evaluated the level of consciousness twice per day. SAE was defined as cerebral dysfunction in the presence of sepsis that fulfilled the exclusion criteria. The infection biochemical indicators, Glasgow coma scale (GCS) score, acute physiology and chronic health evaluation score II, serum NSE and S100β were newly measured or evaluated for SAE patients. Finally, hospital mortality, bacteriological categories, length of ICU stay and length of hospital stay were also recorded for all enrolled patients. The data was analyzed with the Chi square test, two-sample t test or Mann–Whitney U test between two groups. The correlation between two factors was analyzed using the Pearson or Spearman analysis. Receiver operating characteristic (ROC) curves were used to determine the ability of S100β and NSE in diagnosing SAE and predicting the hospital mortality. In addition, cut-off points were obtained from the curves to determine the highest sum of sensitivity and specificity. Of 112 enrolled patients, 48 patients were diagnosed with SAE. The serum S100β and NSE concentrations in SAE patients were both significantly higher than in non-SAE patients 0.306 (IQR 0.157–0.880) μg/L vs. 0.095 (IQR 0.066–0.177) μg/L, 24.87 (IQR 31.73–12.73) ng/mL vs. 15.49 (IQR 9.88–21.46) ng/mL, P < 0.01]. GCS scores were related more closely to S100β than NSE (−0.595 vs. −0.337). S100β levels of 0.131 μg/L diagnosed SAE with 67.2 % specificity and 85.4 % sensitivity in the ROC curve, the area under the curve was 0.824 (95 % confidence interval 0.750–0.898). NSE levels of 24.15 ng/mL diagnosed SAE with 82.8 % specificity and 54.2 % sensitivity, and the area under the curve was 0.664 (95 % confidence interval 0.561–0.767). In addition, the area under the curve for S100β for predicting hospital mortality was larger than for NSE (0.730 vs. 0.590). Serum S100β concentrations in SAE patients were significantly higher than in non-SAE patients. These may be related to the severity of SAE and may predict the outcome of sepsis. The efficacy and sensitivity of serum S100β in diagnosing SAE were high, but it had a low specificity. Moreover, compared to NSE, serum S100β was better for both diagnosing SAE and predicting the outcome of sepsis.

Similar content being viewed by others
Abbreviations
- SAE:
-
Sepsis-associated encephalopathy
- NSE:
-
Neuron-specific enolase
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- ANOVA:
-
Analysis of variance
- GCS:
-
Glasgow coma scale
- APACHEII score:
-
Acute physiology and chronic health evaluation score II
- PCT:
-
Procalcitonin
- WBC:
-
White blood cell count
- CRP:
-
C-reactive protein
References
Gofton TE, Young GB (2012) Sepsis-associated encephalopathy. Nat Rev Neurol 8:557–566
Zhang LN, Wang XT, Ai YH, Guo QL, Huang L, Liu ZY, Yao B (2012) Epidemiological features and risk factors of sepsis-associated encephalopathy in intensive care unit patients: 2008–2011. Chin Med J (Engl) 125:828–831
Comim CM, Constantino LC, Barichello T, Streck EL, Quevedo J, Dal-Pizzol F (2009) Cognitive impairment in the septic brain. Curr Neurovasc Res 6:194–203
Piazza O, Cotena S, Robertis ED, Caranci F, Tufano R (2009) Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochem Res 34:1289–1292
Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, de la Grandmaison GL, Aboab J, Gray F, Menone D, Annane D (2007) Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med 33:798–806
Garnier Y, Frigiola A, Li VG, Florio P, Frulio R, Berger R, Alm S, von Duering MU, Coumans AB, Reis FM, Petraglia F, Hassart TH, Abella R, Mufeed H, Gazzolo D (2009) Increased maternal/fetal blood S100β levels following systemic endotoxin administration and periventricular white matter injury in preterm fetal sheep. Reprod Sci 16:758–766
Young GB, Bolton CF, Archibald YM, Austin TW, Wells GA (1992) The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol 9:145–152
Sharshar T, Poron F, Gray F, Rahael JC, Gajdos P, Annane D (2002) Multifocal necrotizing leukoencephalopathy in septic shock. Crit Care Med 30:2371–2375
Rosengarten B, Hecht M, Auch D, Ghofrani HA, Schermuly RT, Grimminger F, Kaps M (2007) Microcirculatory dysfunction in the brain precedes changes in evoked potentials in endotoxin-induced sepsis syndrome in rats. Cerebrovasc Dis 23:140–147
Pelinka LE, Toegel E, Mauritz W, Redl H (2003) Serum S100B: a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock 19:195–200
González-García S, González-Quevedo A, Fernández-Concepción O, Peña-Sánchez M, Menéndez-Saínz C, Hernández-Díaz Z, Arteche-Prior M, Pando-Cabrera A, Fernández-Novales C (2012) Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem 45:1302–1307
Derwall M, Stoppe C, Brücken D, Rossaint R, Fries M (2009) Changes in S-100 protein serum levels in survivors of out-of-hospital cardiac arrest treated with mild therapeutic hypothermia: a prospective, observational study. Critical Care. doi:10.1186/cc7785
Hsu AA, Fenton K, Weinstein S, Carpenter J, Dalton H, Bell MJ (2008) Neurological injury markers in children with septic shock. Pediatr Crit Care Med 9:245–251
Larsson A, Lipcsey M, Sjölin J, Hansson LO, Eriksson MB (2005) Slight increase of serum S-100β during porcine endotoxemic shock may indicate blood-brain barrier damage. Anesth Analg 101:1465–1469
Lipcsey M, Olovsson M, Larsson E, Einarsson R, Qadhr GA, Sjölin J, Larsson A (2010) The brain is a source of S100B increase during endotoxemia in the pig. Anesth Analg 110:174–180
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Heizmann CW, Fritz G, Schäfer BW (2002) S100 proteins: structure, functions and pathology. Front Biosci 7:d1356–d1368
Zimmer DB, Van Eldick LJ (1986) Identification of a molecular target for the calcium-modulated protein S100: fructose-1,6-bisphosphate aldolase. J Biol Chem 261:11424–11428
Sorci G, Agneletti AL, Bianchi R, Donato R (1998) Association of S100B with intermediate filaments and microtubules in glial cells. Biochem Biophys Acta 1448:277–289
Xiong ZG, O’Hanlon D, Becker LE, Roder J, MacDonald JF, Marks A (2000) Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp Cell Res 257:281–289
Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H (2000) Coregulation of neurite out-growth and cell survival by amphoterin and S100 proteins through RAGE activation. J Biol Chem 275:40096–40105
Sorci G, Riuzzi F, Agneletti AL, Marchetti C, Donato R (2004) S100B causes apoptosis in a myoblast cell line in a RAGE-independent manner. J Cell Physiol 199:274–283
Eidelman LA, Putterman D, Putterman C, Sprung CL (1996) The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA 275:470–473
Hamed SA, Hamed EA, Abdella MM (2009) Septic encephalopathy: relationship to serum and cerebrospinal fluid levels of adhesion molecules, lipid peroxides and S-100B protein. Neuropediatrics 40:66–72
Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ (2008) TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int 52:447–456
Pelinka LE, Harada N, Szalay L, Jafarmadar M, Redl H, Bahrami S (2004) Release of S100β differs during ischemia and reperfusion of the liver, the gut, and the kidney in rats. Shock 21:72–76
Goncalves CA, Leite MC, Guerra MC (2010) Adipocytes as an important source of serum S100β and possible roles of this protein in adipose tissue. Cardiovasc Psychiatry Neurol. doi:10.1155/2010/790431
Piazza O, Russo E, Cotena S, Esposito G, Tufano R (2007) Elevated S100β levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth 99:518–521
Gazzolo D, Bruschettini M, Lituania M, Serra G, Bonacci W, Michetti F (2001) Increased urinary S100β protein as an early indicator of intraventricular hemorrhage in preterm infants: correlation with the grade of hemorrhage. Clin Chem 47:1836–1838
Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, Espinoza J (2007) The calcium binding protein, S100β, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 35:385–393
Mussack T, Briegel J, Schelling G, Jochum M (2005) Hemofiltration does not influence early S-100β serum levels in septic shock patients receiving stress doses of hydrocortisone or placebo. Eur J Med Res 10:81–87
Acknowledgments
The authors thank Zhao-hui CHEN, the senior laboratory technician in the nuclear medicine department of the Xiangya hospital, for helping in measuring the concentration of serum S100β and NSE. We also thank Lin-yong XU, professor in the college of public hygiene of the Xiangya medical University for supervising the statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Bo Yao and Li-Na Zhang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yao, B., Zhang, LN., Ai, YH. et al. Serum S100β is a Better Biomarker than Neuron-Specific Enolase for Sepsis-Associated Encephalopathy and Determining Its Prognosis: A Prospective and Observational Study. Neurochem Res 39, 1263–1269 (2014). https://doi.org/10.1007/s11064-014-1308-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1308-0