Abstract
Purpose
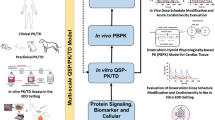
The mechanisms underlying doxorubicin cytotoxicity and cardiotoxicity were broadly explored but remain incompletely understood. A multiscale physiologically-based pharmacokinetic (PBPK) model was developed to assess doxorubicin dispositions at levels of system, tissue interstitial, cell, and cellular organelles. This model was adopted to explore the mechanisms-of-action/toxicity of doxorubicin in humans.
Methods
The PBPK model was developed by analyzing data from mice and the model was verified by scaling up to predict doxorubicin multiscale dispositions in rats and humans. The multiscale dispositions of doxorubicin in human heart and tumors were explicitly simulated to elucidate the potential mechanisms of its cytotoxicity and cardiotoxicity.
Results
The developed PBPK model was able to adequately describe doxorubicin dispositions in mice, rats and humans. In humans, prolonged infusion, a dosing regimen with less cardiotoxicity, was predicted with substantially reduced free doxorubicin concentrations at human heart interstitium, which were lower than the concentrations associated with oxidative stress. However, prolonged infusion did not reduce doxorubicin-DNA adduct at tumor nucleus, consistent with clinical observations that prolonged infusion did not compromise anti-tumor effect, indicating that one primary anti-tumor mechanism was DNA torsion.
Conclusions
A multiscale PBPK model for doxorubicin was developed and further applied to explore its cytotoxic and cardiotoxic mechanisms.







Similar content being viewed by others
References
Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JA, Saffi J. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90(9):2063–76.
Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp. 2009;57(6):435–45.
Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011;37(4):300–11.
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16(25):3267–85.
Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–41.
Yang F, Teves SS, Kemp CJ, Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014;1845(1):84–9.
Johansen PB. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother Pharmacol. 1981;5(4):267–70.
Formelli F, Carsana R, Pollini C. Comparative pharmacokinetics and metabolism of doxorubicin and 4-demethoxy-4'-O-methyldoxorubicin in tumor-bearing mice. Cancer Chemother Pharmacol. 1986;16(1):15–21.
Soininen SK, Vellonen KS, Heikkinen AT, Auriola S, Ranta VP, Urtti A, et al. Intracellular PK/PD relationships of free and liposomal doxorubicin: quantitative analyses and PK/PD modeling. Mol Pharm. 2016;13(4):1358–65.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37.
Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157(6):907–21.
Gustafson DL, Rastatter JC, Colombo T, Long ME. Doxorubicin pharmacokinetics: macromolecule binding, metabolism, and excretion in the context of a physiologic model. J Pharm Sci. 2002;91(6):1488–501.
Dubbelboer IR, Lilienberg E, Sjögren E, Lennernäs H. A model-based approach to assessing the importance of intracellular binding sites in doxorubicin disposition. Mol Pharm. 2017;14(3):686–98.
Gustafson DL, Thamm DH. Pharmacokinetic modeling of doxorubicin pharmacokinetics in dogs deficient in ABCB1 drug transporters. J Vet Intern Med. 2010;24(3):579–86.
Reddy LH, Murthy RS. Pharmacokinetics and biodistribution studies of doxorubicin loaded poly(butyl cyanoacrylate) nanoparticles synthesized by two different techniques. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(2):161–6.
Lu WL, Qi XR, Zhang Q, Li RY, Wang GL, Zhang RJ, et al. A pegylated liposomal platform: pharmacokinetics, pharmacodynamics, and toxicity in mice using doxorubicin as a model drug. J Pharmacol Sci. 2004;95(3):381–9.
Rodionov N. Graph digitizer version 1.9. (2000) http://www.geocities.com/graphdigitizer
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–70.
Kawai R, Mathew D, Tanaka C, Rowland M. Physiologically based pharmacokinetics of cyclosporine a: extension to tissue distribution kinetics in rats and scale-up to human. J Pharmacol Exp Ther. 1998;287(2):457–68.
Maniez-Devos DM, Baurain R, Trouet A, Lesne M. Doxorubicin pharmacokinetics in the rabbit. Aust J Pharm. 1985;16(2):159–69.
El-Kareh AW, Secomb TW. Two-mechanism peak concentration model for cellular pharmacodynamics of doxorubicin. Neoplasia. 2005;7(7):705–13.
Hendriks BS, Reynolds JG, Klinz SG, Geretti E, Lee H, Leonard SC, et al. Multiscale kinetic modeling of liposomal doxorubicin delivery quantifies the role of tumor and drug-specific parameters in local delivery to tumors. CPT Pharmacometrics Syst Pharmacol. 2012;1:e15.
Becelli R, Renzi G, Morello R, Altieri F. Intracellular and extracellular tumor pH measurement in a series of patients with oral cancer. J Craniofac Surg. 2007;18(5):1051–4.
Yang M, Chan HL, Lam W, Fong WF. Cytotoxicity and DNA binding characteristics of dextran-conjugated doxorubicins. Biochim Biophys Acta. 1998;1380(3):329–35.
Mross K, Maessen P, van der Vijgh WJ, Gall H, Boven E, Pinedo HM. Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J Clin Oncol. 1988;6(3):517–26.
Benjamin RS, Riggs CE Jr, Bachur NR. Pharmacokinetics and metabolism of adriamycin in man. Clin Pharmacol Ther. 1973;14(4):592–600.
Arnold RD, Mager DE, Slack JE, Straubinger RM. Effect of repetitive administration of doxorubicin-containing liposomes on plasma pharmacokinetics and drug biodistribution in a rat brain tumor model. Clin Cancer Res. 2005;11(24 Pt 1):8856–65.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci. 1986;75(11):1028–40.
van Dalen EC, van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2016;3:CD005008.
Speth PA, van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet. 1988;15(1):15–31.
Gunvén P, Theve NO, Peterson C. Serum and tissue concentrations of doxorubicin after IV administration of doxorubicin or doxorubicin-DNA complex to patients with gastrointestinal cancer. Cancer Chemother Pharmcol. 1986;17(2):153–6.
Rossi C, Gasparini G, Canobbio L, Galligioni E, Volpe R, Candiani E, et al. Doxorubicin distribution in human breast cancer. Cancer Treat Rep. 1987;71(12):1221–6.
Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11(19 Pt 1):6944–9.
Shapira J, Gotfried M, Lishner M, Ravid M. Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen. A prospective randomized evaluation. Cancer. 1990;65(4):870–3.
Bi Y, Deng J, Murry DJ, An G. A whole-body physiologically based pharmacokinetic model of Gefitinib in mice and scale-up to humans. AAPS J. 2016;18(1):228–38.
An G, Morris ME. A physiologically based pharmacokinetic model of mitoxantrone in mice and scale-up to humans: a semi-mechanistic model incorporating DNA and protein binding. AAPS J. 2012;14(2):352–64.
Hudachek SF, Gustafson DL. Physiologically based pharmacokinetic model of lapatinib developed in mice and scaled to humans. J Pharmacokinet Pharmacodyn. 2013;40(2):157–76.
Jamieson D, Boddy AV. Pharmacogenetics of genes across the doxorubicin pathway. Expert Opin Drug Metab Toxicol. 2011;7(10):1201–10.
Lal S, Wong ZW, Sandanaraj E, Xiang X, Ang PC, Lee EJ, et al. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99(4):816–23.
ACKNOWLEDGMENTS and DISCLOSURES
This work was supported by FDA (U01 FD005206) and NIH (GM119661). The views expressed in this article are those of the authors and not necessarily those of the Food and Drug Administration (FDA). We appreciate the discussion and suggestions with Dr. William Jusko from State University of New York at Buffalo. There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
He, H., Liu, C., Wu, Y. et al. A Multiscale Physiologically-Based Pharmacokinetic Model for Doxorubicin to Explore its Mechanisms of Cytotoxicity and Cardiotoxicity in Human Physiological Contexts. Pharm Res 35, 174 (2018). https://doi.org/10.1007/s11095-018-2456-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2456-8