Abstract
Absent in melanoma 2 (AIM2) is a recently recognized cytoplasmic receptor which could sense cytoplasmic double-stranded DNA (dsDNA). After AIM2 detects the presence of parasitic nucleic acids (dsDNA) derived from invasive bacteria or viral genomes (for example, vaccinia virus and cytomegalovirus) within infected cells, AIM2 inflammasome could be formed. The formed AIM2 inflammasome could induce innate immune response and increase expressions of IL-1β and IL-18. Hepatitis B virus (HBV) is a hepatotropic, non-cytopathic double-stranded DNA virus. The immune response to viral antigens or virus is thought to be responsible for both liver damage and viral clearance in patients with HBV infection. However, there are no reports about whether AIM2 inflammasome exists in hepatocytes. In the present study, we investigated the presence and activity of AIM2 inflammasome in human hepatocytes. We found that AIM2 was expressed in cytoplasm of hepatocytes, and IL-18 expression was increased after AIM2 sensed HBV in hepatocytes in vitro. These results showed that AIM2 inflammasome was active in hepatocytes. We also found that hepatic AIM2 expression of chronic hepatitis B (CHB) patients was higher than that of controls. Hepatic AIM2 expression levels were positively correlated to the severity of liver inflammation. IL-18 is already considered to be associated with hepatic injury during HBV infection. In conclusion, we, therefore, believe that AIM2 inflammasome in hepatocytes might play an important role in the development and maintenance of HBV-related hepatitis.
Similar content being viewed by others
Introduction
It is estimated that 2 billion people have been infected with hepatitis virus B (HBV), and 350–400 million people are chronic carries of the virus in the world [1, 2]. More than 0.5–1 million people die from HBV-related end stage liver diseases or HCC every year [2]. HBV infection already becomes the tenth leading cause of death worldwide [1]. However, the mechanism of pathogenesis after HBV infection is still not thoroughly been elucidated. The immune response to viral antigens or virus is thought to be responsible for both liver damage and viral clearance in patients with HBV infection, though HBV does not have directly cytopathic effect on the hepatocytes [3]. However, how the host immune system senses HBV is not fully known.
HBV is a hepatotropic, non-cytopathic DNA virus. Upon infection of the hepatocytes, the genomic DNA of HBV is transferred to the cell nucleus, where the partially double-stranded DNA is converted into covalently closed circular DNA (cccDNA). Then cccDNA is transcribed into pregenomic RNA. Pregenomic RNA enters into cytoplasm and is reversely transcribed into dsDNA in the cytoplasm of hepatocytes.
Absent in melanoma 2 (AIM2), a recently recognized cytoplasmic receptor, could detect the presence of parasitic nucleic acids derived from invasive bacteria or viral genomes within the infected cells [4]. After AIM2 recognizing double-stranded DNA (dsDNA), the PYD domain of AIM2 associates with the adapter molecule apoptosis-associated speck-like protein containing a CARD (ASC). As a result, AIM2 inflammasome is formed, which could activate caspase-1 [5]. Caspase-1 is responsible for the processing and secretion of IL-1β [6] and IL-18 [7], and induces a form of cell death called pyroptosis [8]. Finally, the detection of cytoplasmic dsDNA by AIM2 triggers inflammatory responses. Furthermore, it is reported that AIM2 is involved in the pathogenesis of inflammatory bowel disease [9], psoriasis [10], and other immune diseases.
It is reported that hepatocytes express ASC [11]. Furthermore, hepatocytes could express TNF-α [12], IL-32 [13], and other inflammatory cytokines or chemokines after HBV infection. Recently, Han et al. [14] reports that AIM2 expression is increased in HBV-infected liver, and hepatic AIM2 expression levels are positively correlated with the expression of caspase-1, IL-18 and liver inflammation in CHB patients. Taken together, we suggested that AIM2, a cytoplasmic receptor, might be in involved in the detection of HBV in hepatocytes and be associated with inflammatory response after HBV infection. So we assayed the expression of AIM2 and the activity of AIM2 inflammasome in hepatocytes in the present study, in order to elucidate whether AIM2 recognized and responded to HBV in vitro.
Materials and methods
Subjects
Forty-three patients (31 male, 12 female; age range 30–42 years, average 36 ± 5) with chronic HBV infection were included in this study. Each subject had undergone a percutaneous liver biopsy at the 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China. The standards for diagnoses of CHB have been detailedly described [15, 16]. Patients with antibodies against HIV or other forms of chronic liver disease were excluded. Five normal non-HBV-infected liver tissues from macroscopically normal areas during liver hemangioma resection were used as controls. The demographic, histological, and biochemical characteristics of the enrolled 43 CHB patients are summarized in Table 1. The study was approved by the Human Ethics Committee of The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China. Informed written consent was obtained from each patient in the study.
Reagents
The antibody against AIM2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Human IL-18 ELISA kit and human IL-1β ELISA kit were purchased from R&D systems (R&D, MN). Caspase-1 inhibitor, Z-YVAD-FMK was purchased from BioVision Incorporated (BioVision, CA).
Immunohistochemistry and image analysis
Immunohistochemistry was used to assay hepatic AIM2 expression. The relative mean density (integrated optical density sum/positive area sum) of all the diaminobenzidine-stained areas of each photo was measured to determine hepatic AIM2 expression. Immunohistochemical staining and image analysis was performed as described previously in detail [17].
RNA extraction and real-time PCR
AIM2 mRNA expression in liver tissues was assessed by quantitative real-time PCR. AIM2 specific primers were as follows [18]: forward primer, 5′-TCAAGCTGAAATGAGTCCTGC-3′, reverse primer, 5′-CTTGGGTCTCAAACGTGAAGG-3′. RNA extraction and real-time PCR were performed as described previously in detail [17]. The details were as follows: total RNA of hepatic tissues was extracted by using TRIzol (TaKaRa). PrimeScript™ II 1st Strand cDNA Synthesis Kit was used to synthesize cDNA (TaKaRa). Subsequently, cDNA was submitted to PCR in the presence of the SYBR Premix Ex Taq (TaKaRa) and real-time PCR detection machine ABI7500 PRISM (Applied Biosystems, Sunnyvale, CA). The internal control was the house-keeping gene β-actin. All operations complied with the manufacturer’s protocol.
Cell line and cell culture
HepG2 (human hepatocellular cell line) cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % FBS, 100 μg/ml streptomycin, and 100 μg/ml penicillin at 37 °C in a 5 % CO2 incubator.
Transient transfection
HepG2 (human hepatocellular cell line) cells were plated at a density of 4 × 105 cell per 6-well plate or 24-well plate. After 24 h, cells were transfected with the full-length gene of HBV (pBlue-HBV) plasmid or the empty plasmid (pBlue-SK). The construction-containing HBV gene was kindly provided by Prof. Guanxin Shen (Department of Immunology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China). esiRNA targeting human AIM2 (esiRNA1) and esiRNA-RnLuc (unrelated siRNA, used as control) were purchased from Sigma-Aldrich (SIGMA, MO). Lipofectamine 3000 was used to transfect plasmid or esiRNA into cells.
ELISA and western blotting
HepG2 cells were transfected with the vector containing HBV gene. Forty-eight hours later, the culture supernatants were harvested, and the amounts of IL-1β or IL-18 present in culture supernatants were measured by ELISA (R&D, MA) according to the manufacturer’s instructions. Then HepG2 cells were co-transfected with esiRNA targeting human AIM2 (esiRNA1) or esiRNA-RnLuc, the vector containing HBV gene. Forty-eight hours later, the culture supernatants were harvested and the amounts of IL-1β or IL-18 present in culture supernatants were measured by ELSIA. Furthermore, Cytoplasmic proteins were extracted, and AIM2 protein was detected by Western blotting. Western blotting was performed as described previously in detail [13]. Subsequently, HepG2 cells were transfected with the vector containing HBV gene. Then caspase-1 inhibitor, Z-YVAD-FMK, was added or not added to the culture, respectively. After 48 h, the amounts of IL-18 present in culture supernatants were measured by ELISA.
Laboratory Tests
Laboratory tests were previously described [19]. In brief, routine automated analysis system was used to detect liver biochemistry (Beckman Coulter, CA). Chemiluminescent micro particle enzyme immunoassay was used to detect HBV serological markers (Abbott, Chicago, IL).
Statistics
Data were expressed as mean ± SD. The means among groups were analyzed using student’s t test or one-way ANOVA. All statistical analyses were performed using SPSS v17.00 statistical analysis software (SPSS Inc, Chicago, IL). Differences were considered statistically significant at a value of P < 0.05.
Results
AIM2 mRNA expression was increased in liver tissues of CHB patients
Hepatic AIM2 mRNA expression was detected in each sample. As shown in Fig. 1a, AIM2 mRNA expression in liver tissues of CHB patients was significantly higher than that in controls (P < 0.05). Moreover, hepatic AIM2 mRNA expression levels were positively correlated to the severity of liver inflammation. Compared with CHB patients with liver inflammation grade 1 or grade 2, hepatic AIM2 mRNA expression levels were significantly increased in CHB patients with liver inflammation grade 3/4 (Fig. 1b) (P < 0.05).
AIM2 protein expression was increased in liver tissues of CHB patients
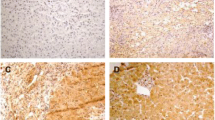
As shown in Fig. 2, immunohistochemical staining demonstrated that the expression of AIM2 protein occurred nearly in all liver cells, and the localization of AIM2 was in cytoplasm of hepatocytes. The expression of AIM2 in liver tissue from CHB patients was higher than that of controls (Fig. 2). Furthermore, the intensity of hepatic AIM2 staining gradually increased with the severity of liver inflammation (Fig. 2a–e). Isotype IgG was used as a negative control in liver tissues of CHB (Fig. 2f). Subsequently, we used Image-Pro Plus 6.0 software to analyze the relative mean density of all the diaminobenzidine-stained areas of each photo. As shown in Fig. 2g, compared with that in inflammation grade 1 or grade 2, the relative mean density of hepatic AIM2 staining in inflammation grade 3/4 was significantly augmented (P < 0.05).
Expression of hepatic AIM2 protein in liver biopsy sections. AIM2 expression in liver tissues was analyzed by immunohistochemical staining. AIM2 positivity was found nearly in all hepatocytes (×200). As shown in b–e, the intensity of immunohistochemical staining was gradually increased with the severity of liver inflammation (×200). f, Isotype IgG was used as a negative control in liver tissues of CHB. Figure 2g showed that relative mean density of hepatic AIM2 staining in inflammation grade 3/4 was significantly higher than that of inflammation grade 1 or 2 (P < 0.05)
IL-18 expression was increased after human hepatocytes were transfected with the plasmid pBlue-HBV
In order to assay the activity of AIM2 inflammasome, HepG2 cells were transfected with the plasmid pBlue-HBV (the full-length gene of HBV). Forty-eight hours later, the culture supernatants were harvested, HBV DNA levels were assayed by using real-time PCR and the release of IL-1β or IL-18 was measured by ELISA. We found that HBV DNA levels in supernatants of cells transfected with the plasmid pBlue-HBV were to 103 or >103 copies/ml. HBV DNA levels was not detected in supernatants of cells transfected with the empty vector (pBlue-SK). Figure 3a showed that compared with cells transfected with the empty vector, the release of IL-18 was significantly enhanced after these cells were transfected with the plasmid pBlue-HBV, but the release of IL-1β was not significantly increased. Furthermore, the plasmid pBlue-HBV induced the release of IL-18 from hepatocytes in a dose-dependent manner (Fig. 3b).
IL-18 release of HepG2 cells after the transfection of the plasmid pBlue-HBV. After 48 h of transfection, the culture supernatants were harvested and the amounts of IL-18 (A-a) or IL-1β (A-b) present in culture supernatants were measured by ELISA. b the release of IL-18 from HepG2 cells transfected with different concentrations of the plasmid pBlue-HBV. Results showed that HBV induced IL-18 expression in a dose-dependent manner. The results were an experiment that was set up as a triplicate
IL-18 expression was inhibited by esiRNA targeting human AIM2 (esiRNA1)
To identify that the increased release of IL-18 was mediated by AIM2, different concentrations of esiRNA1, and the plasmid pBlue-HBV were co-transfected to HepG2 cells. Forty-eight hours later, the culture supernatants were harvested and the cytoplasmic proteins were extracted, respectively. The release of IL-18 in the culture supernatants was measured by ELISA. As shown in Fig. 4a, inhibition of AIM2 by esiRNA1 reduced the release of IL-18 from HepG2 cells. AIM2 protein expression in the cytoplasmic proteins was detected by Western blotting. As shown in Fig. 4b, AIM2 protein expression was gradually inhibited by esiRNA1.
Inhibition of IL-18 expression from HepG2 cells by esiRNA targeting human AIM2 (esiRNA1). HepG2 cells were co-transfected with different concentrations of esiRNA1 and the plasmid pBlue-HBV. After 48 h, the culture supernatants were harvested and the cytoplasmic proteins were extracted, respectively. a The release of IL-18 in the culture supernatants was measured by ELISA. b AIM2 protein expression in the cytoplasmic proteins was detected by Western blotting. The results were an experiment that was set up as a triplicate
IL-18 expression was inhibited by Z-YVAD-FMK of caspase-1 inhibitor
Caspase-1 is activated by AIM2 inflammasome. The activated caspase-1 is responsible for the processing and secretion of IL-18. In order to evaluate the activity of AIM2 inflammasome, Z-YVAD-FMK, a caspase-1 inhibitor, was added into the cultures when HepG2 cells were transfected with the plasmid pBlue-HBV. After 48 h, the culture supernatants were collected and the expression level of IL-18 was measured by ELISA. As shown in Fig. 5, the expression of IL-18 decreased in the presence of caspase-1 inhibitor Z-YVAD-FMK.
Inhibition of IL-18 release from HepG2 cells in the presence of Z-YVAD-FMK, a caspase-1 inhibitor. Different concentrations of Z-YVAD-FMK were used to treat HepG2 cells after the transfection of the plasmid pBlue-HBV. Forty-eight hours later, IL-18 release from HepG2 cells was assayed by ELISA. The results were an experiment that was set up as a triplicate
Discussion
In this report, we found that hepatic AIM2 expression in CHB patients was significantly higher than that in controls. Hepatic AIM2 expression levels were positively correlated to the severity of liver inflammation. Furthermore, the activated AIM2 inflammasome triggered IL-18 release in response to HBV in HepG2 cells. IL-18 is a leukocyte chemotactic and activating cytokine [20]. IL-18 induces the expressions of IL-8, TNF-α, and other inflammatory cytokines [21]. IL-18 is associated with hepatic injury by increasing FasL expression during HBV infection [22]. So we think that activation of AIM2 inflammasome in hepatocytes might play an important role in the development and maintenance of HBV-related hepatitis.
Several researches [23, 24] find that foreign cytoplasmic dsDNA could induce innate immune response. Recently, it is reported that AIM2 could bind to vaccinia virus [5] and cytomegalovirus [25], and trigger innate immune response. Our results further demonstrated that the sensing of HBV by AIM2 might induce innate immunity. Both innate immune response [26] and adaptive immunity [27] are involved in liver damage after HBV infection. So we believe that AIM2 might be associated with the development and maintenance of HBV-related hepatitis.
It is reported [28] that hepatocytes themselves might play active role in innate immune response to virus infection. It is reported that hepatocytes express ASC [11] and caspase-1 [29]. It is reported [30] that IL-1β could be expressed by hepatocytes in response to hepatitis C virus (HCV) infection. The mechanism might be as follows: NACHT, LRR, and PYD domains-containing protein 3 (NALP3), one of nucleotide-binding-domain leucine-rich-repeat-containing molecules which could sense RNA in cytoplasm, was expressed by hepatocytes. The recognition of HCV by NALP3 might recruit ASC for the assembly of NALP3-inflammasome complex. The NALP3-inflammasome complex activated caspase-1. Then caspase-1 activated IL-1β. Our results found that AIM2 was expressed in hepatocytes and the activated AIM2 inflammasome triggered IL-18 release in response to HBV. Taken together, a new signal pathway, which could sense foreign cytoplasmic nucleic acids and induce immune response, might exist in hepatocytes and be involved in the pathogenesis of virus-related hepatitis.
Previous research [22] demonstrates that HBx protein could induce IL-18 expression in hepatocytes, but the mechanism of HBx-induced IL-18 expression is still not elucidated. In the present study, we found that the activated AIM2 inflammasome triggered IL-18 release in response to HBV in hepatocytes. This might explain the increased IL-18 expression after HBV infection. Moreover, our results could further help us to learn how HBV-related liver injury occurred after HBV infection.
In the present research, immunohistochemical studies showed cytoplasmic localization of AIM2 in hepatocytes. Moreover, hepatic AIM2 expression levels were positively correlated to the severity of liver inflammation. Our results were in accordance with the research of Han et al. [14]. However, we also found that the activated AIM2 inflammasome triggered IL-18 release in response to HBV in hepatocytes in vitro. These results further demonstrated AIM2 might play a vital role in the pathogenesis of HBV-related hepatitis. In the present study, IL-18 expression induced by HBV significantly decreased after AIM2 was interfered by esiRNA1. IL-18 expression was also inhibited by Z-YVAD-FMK of caspase-1 inhibitor. So we believe that AIM2 and AIM2 inflammasome might be new potential targets for prevention and treatment of HBV-related hepatitis. Next we will investigate roles of AIM2 and AIM2 inflammasome in animal model of hepatitis B.
HBV and proteins encoded by HBV genome could induce expressions of many proteins, such as IL-32 [13] and IP-10 [31]. Recently, it is found that AIM2 expression could be regulated by Neisseria meningitides [32]. In the present study, we found that the expression of AIM2 in liver tissue from CHB patients was significantly higher than that in controls. Taken together, we supposed that HBV or proteins encoded by HBV genome could regulate the expression of AIM2. However, the detailed signal transduction pathway is unknown and needs further investigation.
In sum, the expression of AIM2 in liver tissues from CHB patients was significantly higher than that in controls. Hepatic AIM2 expression levels were positively correlated to the severity of liver inflammation. Furthermore, the activated AIM2 inflammasome triggered IL-18 release in response to pBlue-HBV in HepG2 cells. We, therefore, believe that AIM2 might play an important role in the development and maintenance of HBV-related hepatitis. This might also be mechanism that the body defends itself against pathogen infection and clears the infected pathogen.
References
C. Trepo, H.L. Chan, A. Lok, Hepatitis B virus infection. Lancet 384, 2053–2063 (2014)
European Association For The Study Of The Liver, EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 57, 167–185 (2012)
M.K. Maini, C. Boni, C.K. Lee, J.R. Larrubia, S. Reignat, G.S. Ogg, A.S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N.V. Naoumov, C. Ferrari, A. Bertoletti, The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191, 1269–1280 (2000)
R.L. Brunette, J.M. Young, D.G. Whitley, I.E. Brodsky, H.S. Malik, D.B. Stetson, Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J. Exp. Med. 209, 1969–1983 (2012)
V. Hornung, A. Ablasser, M. Charrel-Dennis, F. Bauernfeind, G. Horvath, D.R. Caffrey, E. Latz, K.A. Fitzgerald, AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009)
D.P. Cerretti, C.J. Kozlosky, B. Mosley, N. Nelson, K. Van Ness, T.A. Greenstreet, C.J. March, S.R. Kronheim, T. Druck, L.A. Cannizzaro, A. Et, Molecular cloning of the interleukin-1 beta converting enzyme. Science 256, 97–100 (1992)
M. Keller, A. Ruegg, S. Werner, H.D. Beer, Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008)
T. Strowig, J. Henao-Mejia, E. Elinav, R. Flavell, Inflammasomes in health and disease. Nature 481, 278–286 (2012)
J.M. Kim, Inflammatory bowel diseases and inflammasome. Korean J Gastroenterol 58, 300–310 (2011)
Y. Dombrowski, M. Peric, S. Koglin, C. Kammerbauer, C. Goss, D. Anz, M. Simanski, R. Glaser, J. Harder, V. Hornung, R.L. Gallo, T. Ruzicka, R. Besch, J. Schauber, Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 3, 38r–82r (2011)
J. Masumoto, S. Taniguchi, J. Nakayama, M. Shiohara, E. Hidaka, T. Katsuyama, S. Murase, J. Sagara, Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J. Histochem. Cytochem. 49, 1269–1275 (2001)
C.Y. Yang, T.H. Kuo, L.P. Ting, Human hepatitis B viral e antigen interacts with cellular interleukin-1 receptor accessory protein and triggers interleukin-1 response. J. Biol. Chem. 281, 34525–34536 (2006)
X. Pan, H. Cao, J. Lu, X. Shu, X. Xiong, X. Hong, Q. Xu, H. Zhu, G. Li, G. Shen, Interleukin-32 expression induced by hepatitis B virus protein X is mediated through activation of NF-kappaB. Mol. Immunol. 48, 1573–1577 (2011)
Y. Han, Z. Chen, R. Hou, D. Yan, C. Liu, S. Chen, X. Li, W. Du, Expression of AIM2 is correlated with increased inflammation in chronic hepatitis B patients. Virol. J. 12, 129 (2015)
X.W. Xu, M.H. Lu, D.M. Tan, Association between tumour necrosis factor gene polymorphisms and the clinical types of patients with chronic hepatitis B virus infection. Clin. Microbiol. Infect. 11, 52–56 (2005)
M. Han, W. Yan, W. Guo, D. Xi, Y. Zhou, W. Li, S. Gao, M. Liu, G. Levy, X. Luo, Q. Ning, Hepatitis B virus-induced hFGL2 transcription is dependent on c-Ets-2 and MAPK signal pathway. J. Biol. Chem. 283, 32715–32729 (2008)
Q. Xu, X. Pan, X. Shu, H. Cao, X. Li, K. Zhang, J. Lu, Y. Zou, X. Li, H. Liu, Y. Zhang, D. Yang, Q. Ning, G. Shen, G. Li, Increased interleukin-32 expression in chronic hepatitis B virus-infected liver. J. Infect. 65, 336–342 (2012)
J. Zhen, L. Zhang, J. Pan, S. Ma, X. Yu, X. Li, S. Chen, W. Du, AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1beta, and IL-18. Mediat. Inflamm. 2014, 190860 (2014)
X. Xiang, H. Gui, N. King, L. Cole, H. Wang, Q. Xie, S. Bao, IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol. Cell. Biol. 90, 611–619 (2012)
S. Lebel-Binay, A. Berger, F. Zinzindohoue, P. Cugnenc, N. Thiounn, W.H. Fridman, F. Pages, Interleukin-18: biological properties and clinical implications. Eur. Cytokine Netw. 11, 15–26 (2000)
A.J. Puren, G. Fantuzzi, Y. Gu, M.S. Su, C.A. Dinarello, Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest 101, 711–721 (1998)
M.O. Lee, Y.H. Choi, E.C. Shin, H.J. Kang, Y.M. Kim, S.Y. Jeong, J.K. Seong, D.Y. Yu, H. Cho, J.H. Park, S.J. Kim, Hepatitis B virus X protein induced expression of interleukin 18 (IL-18): a potential mechanism for liver injury caused by hepatitis B virus (HBV) infection. J. Hepatol. 37, 380–386 (2002)
D.B. Stetson, R. Medzhitov, Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24, 93–103 (2006)
K.J. Ishii, C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, S. Akira, A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7, 40–48 (2006)
V.A. Rathinam, Z. Jiang, S.N. Waggoner, S. Sharma, L.E. Cole, L. Waggoner, S.K. Vanaja, B.G. Monks, S. Ganesan, E. Latz, V. Hornung, S.N. Vogel, E. Szomolanyi-Tsuda, K.A. Fitzgerald, The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010)
C. Dunn, M. Brunetto, G. Reynolds, T. Christophides, P.T. Kennedy, P. Lampertico, A. Das, A.R. Lopes, P. Borrow, K. Williams, E. Humphreys, S. Afford, D.H. Adams, A. Bertoletti, M.K. Maini, Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 204, 667–680 (2007)
Z. Zhang, D. Chen, J. Yao, H. Zhang, L. Jin, M. Shi, H. Zhang, F.S. Wang, Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol 122, 173–180 (2007)
S. Preiss, A. Thompson, X. Chen, S. Rodgers, V. Markovska, P. Desmond, K. Visvanathan, K. Li, S. Locarnini, P. Revill, Characterization of the innate immune signalling pathways in hepatocyte cell lines. J Viral Hepat 15, 888–900 (2008)
E.C. Shin, J.M. Ahn, C.H. Kim, Y. Choi, Y.S. Ahn, H. Kim, S.J. Kim, J.H. Park, IFN-gamma induces cell death in human hepatoma cells through a TRAIL/death receptor-mediated apoptotic pathway. Int. J. Cancer 93, 262–268 (2001)
D. Burdette, A. Haskett, L. Presser, S. McRae, J. Iqbal, G. Waris, Hepatitis C virus activates interleukin-1beta via caspase-1-inflammasome complex. J. Gen. Virol. 93, 235–246 (2012)
Y. Zhou, S. Wang, J.W. Ma, Z. Lei, H.F. Zhu, P. Lei, Z.S. Yang, B. Zhang, X.X. Yao, C. Shi, L.F. Sun, X.W. Wu, Q. Ning, G.X. Shen, B. Huang, Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kappaB and increases migration of leukocytes. J. Biol. Chem. 285, 12159–12168 (2010)
U. Gopinathan, R. Ovstebo, O.K. Olstad, B. Brusletto, A.H. Dalsbotten, P. Kierulf, P. Brandtzaeg, J.P. Berg, Global effect of interleukin-10 on the transcriptional profile induced by Neisseria meningitidis in human monocytes. Infect. Immun. 80, 4046–4054 (2012)
Acknowledgments
This study was funded by research grants from National Natural Science Foundation of China (Grant No. 81401306), Doctoral Startup Foundation of the Third Affiliated Hospital of Guangzhou Medical University (No. 2014Y02), and Guangdong Medical Research Foundation (No. A2014318).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Edited by Paul Schnitzler.
Xingfei Pan and Haixia Xu have contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, X., Xu, H., Zheng, C. et al. Human hepatocytes express absent in melanoma 2 and respond to hepatitis B virus with interleukin-18 expression. Virus Genes 52, 445–452 (2016). https://doi.org/10.1007/s11262-016-1327-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-016-1327-9