Abstract
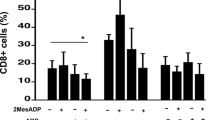
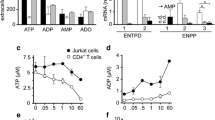
The effects of standard adenosine receptor (AR) agonists and antagonists on the proliferation of human T lymphocytes, unstimulated and phytohemagglutinin-stimulated human peripheral blood lymphocytes (PBL), and Jurkat T cells were investigated. Real-time PCR measurements confirmed the presence of all four AR subtypes on the investigated cells, although at different expression levels. A2A ARs were predominantly expressed in PBL and further upregulated upon stimulation, while malignant Jurkat T cells showed high expression levels of A1, A2A, and A2B ARs. Cell proliferation was measured by [3H]-thymidine incorporation assays. Several ligands, including the subtype-selective agonists CPA (A1), BAY60-6583 (A2B), and IB-MECA (A3), and the antagonists PSB-36 (A1), MSX-2 (A2A), and PSB-10 (A3) significantly inhibited cell proliferation at micromolar concentrations, which were about three orders of magnitude higher than their AR affinities. In contrast, further investigated AR ligands, including the agonists NECA (nonselective) and CGS21680 (A2A), and the antagonists preladenant (SCH-420814, A2A), PSB-1115 (A2B), and PSB-603 (A2B) showed no or only minor effects on lymphocyte proliferation. The anti-proliferative effects of the AR agonists could not be blocked by the corresponding antagonists. The non-selective AR antagonist caffeine stimulated phytohemagglutinin-activated PBL with an EC50 value of 104 μM. This is the first study to compare a complete set of commonly used AR ligands for all subtypes on lymphocyte proliferation. Our results strongly suggest that these compounds induce an inhibition of lymphocyte proliferation and cell death through AR-independent mechanisms.








Similar content being viewed by others
Abbreviations
- ADA:
-
Adenosine deaminase
- AR:
-
Adenosine receptor
- BAY60-6583:
-
2-[6-Amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide
- cAMP:
-
Cyclic AMP
- CGS-21680:
-
(2-p-[2-Carboxyethyl]phenethylamino)-5′-N-ethylcarboxamido-adenosine
- CI-IB-MECA:
-
2-Chloro-N 6-(3-iodobenzyl)-9-[5-(methyl-carbamoyl)-β-d-ribofuranosyl]adenine
- CPA:
-
N 6-Cyclopentyladenosine
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylenediaminetetraacetic acid
- FCS:
-
Fetal calf serum
- GPCR(s):
-
G protein-coupled receptor(s)
- IB-MECA:
-
N 6-(3-Iodobenzyl)-5′-N-methylcarboxamidoadenosine
- MSX-2:
-
3-(3-Hydroxypropyl)-7-methyl-8-(m-methoxystyryl)-1-propargylxanthine
- NECA:
-
5′-N-Ethylcarboxamidoadenosine
- PBL:
-
Peripheral blood lymphocytes
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- PHA:
-
Phytohemagglutinin
- PSB-10:
-
(R)-8-Ethyl-4-methyl-2-(2,3,5-trichlorophenyl)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]purin-5-one
- PSB-36:
-
1-Butyl-8-(3-noradamantanyl)-3-(3-hydroxypropyl)xanthine
- PSB-603:
-
8-[4-[4-(4-Chlorophenyl)piperazine-1-sulfonyl)phenyl]]-1-propylxanthine
- PSB-1115:
-
1-Propyl-8-p-sulfophenylxanthine
- SCID:
-
Severe combined immunodeficiency
- SCH420814:
-
Preladenant
- TNF:
-
Tumor necrosis factor
References
Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63(1):1–34
Mirabet M, Mallol J, Lluis C, Franco R (1997) Calcium mobilization in Jurkat cells via A2b adenosine receptors. Br J Pharmacol 122:1075–1082
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Ernst PB, Garrison JC, Thompson LF (2010) Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol 185(4):1993–1998
Linden J, Cekic C (2012) Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol 32(9):2097–2103
Ferrero ME (2011) Purinoceptors in inflammation: potential as anti-inflammatory therapeutic targets. Front Biosci 16:2172–2186
Burnstock G, Brouns I, Adriaensen D, Timmermans JP (2012) Purinergic signaling in the airways. Pharmacol Rev 64(4):834–868
Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH (2005) Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood 105:4707–4714
Ohta A, Kjaergaard J, Sharma S, Mohsin M, Goel N, Madasu M, Fradkov E, Sitkovsky M (2009) In vitro induction of T cells that are resistant to A2 adenosine receptor-mediated immunosuppression. Br J Pharmacol 156:297–306
Barbieri D, Abbracchio MP, Salvioli S, Monti D, Cossarizza A, Ceruti S, Brambilla R, Cattabeni F, Jacobson KA, Franceschi C (1998) Apoptosis by 2-chloro-2′-deoxy-adenosine and 2-chloro-adenosine in human peripheral blood mononuclear cells. Neurochem Int 32:493–504
Szondy Z (1994) Adenosine stimulates DNA fragmentation in human thymocytes by Ca2+-mediated mechanisms. Biochem J 304:877–885
Apasov SG, Sitkovsky MV (1999) The extracellular versus intracellular mechanisms of inhibition of TCR-triggered activation in thymocytes by adenosine under conditions of inhibited adenosine deaminase. Int Immunol 11:179–189
Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, Mehal WZ, Santamaria P, Shi Y (2007) Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol 179(3):1884–1892
Gelfand EW, Lee JJ, Dosch HM (1979) Selective toxicity of purine deoxynucleosides for human lymphocyte growth and function. Proc Natl Acad Sci U S A 76:1998–2002
Kizaki H, Suzuki K, Tadakuma T, Ishimura Y (1990) Adenosine receptor-mediated accumulation of cyclic AMP-induced T-lymphocyte death through internucleosomal DNA cleavage. J Biol Chem 265:5280–5284
Jackson EK, Gillespie DG, Dubey RK (2011) 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. J Pharmacol Exp Ther 337(2):444–450
Taliani S, La Motta C, Mugnaini L, Simorini F, Salerno S, Marini AM, Da Settimo F, Cosconati S, Cosimelli B, Greco G, Limongelli V, Marinelli L, Novellino E, Ciampi O, Daniele S, Trincavelli ML, Martini C (2010) Novel N2-substituted pyrazolo[3,4-d]pyrimidine adenosine A3 receptor antagonists: inhibition of A3-mediated human glioblastoma cell proliferation. J Med Chem 53:3954–3963
Tanaka Y, Yoshihara K, Tsuyuki M, Kamiya T (1994) Apoptosis induced by adenosine in human leukemia HL-60 cells. Exp Cell Res 213:242–252
Abbracchio MP (1996) P1 and P2 receptors in cell growth and differentiation. Drug Dev Res 39:393–406
Jacobson KA, Hoffmann C, Cattabeni F, Abbracchio MP (1999) Adenosine-induced cell death: evidence for receptor-mediated signalling. Apoptosis 4:197–211
Mlejnek P, Dolezel P, Kosztyu P (2012) P-glycoprotein mediates resistance to A3 adenosine receptor agonist 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-n-methyluronamide in human leukemia cells. J Cell Physiol 227:676–685
Brambilla R, Cattabeni F, Ceruti S, Barbieri D, Franceschi C, Kim YC, Jacobson KA, Klotz KN, Lohse MJ, Abbracchio MP (2000) Activation of the A3 adenosine receptor affects cell cycle progression and cell growth. Naunyn Schmiedebergs Arch Pharmacol 361:225–234
Merighi S, Mirandola P, Milani D, Varani K, Gessi S, Klotz KN, Leung E, Baraldi PG, Borea PA (2002) Adenosine receptors as mediators of both cell proliferation and cell death of cultured human melanoma cells. J Invest Dermatol 119:923–933
Panjehpour M, Karami-Tehrani F (2007) Adenosine modulates cell growth in the human breast cancer cells via adenosine receptors. Oncol Res 16:575–585
Schneider C, Wiendl H, Ogilvie A (2001) Biphasic cytotoxic mechanism of extracellular ATP on U-937 human histiocytic leukemia cells: involvement of adenosine generation. Biochim Biophys Acta 1538:190–205
Fishman P, Bar-Yehuda S, Barer F, Madi L, Multani AS, Pathak S (2001) The A3 adenosine receptor as a new target for cancer therapy and chemoprotection. Exp Cell Res 269:230–236
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Borea PA (2005) A3 adenosine receptor activation inhibits cell proliferation via phosphatidylinositol 3-kinase/Akt-dependent inhibition of the extracellular signal-regulated kinase 1/2 phosphorylation in A375 human melanoma cells. J Biol Chem 280:19516–19526
Blad CC, von Frijtag Drabbe Kunzel JK, de Vries H, Mulder-Krieger T, Bar-Yehuda S, Fishman P, IJzerman AP (2011) Putative role of the adenosine A3 receptor in the antiproliferative action of N 6-(2-isopentenyl)adenosine. Purinergic Signal 7:453–462
Panjehpour M, Castro M, Klotz KN (2005) Human breast cancer cell line MDA-MB-231 expresses endogenous A2B adenosine receptors mediating a Ca2+ signal. Br J Pharmacol 145:211–218
Jackson EK, Ren J, Gillespie DG (2011) 2′,3′-cAMP, 3′-AMP, and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol 301(2):H391–401
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA (2007) Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1a, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol 72(2):395–406
Ma DF, Kondo T, Nakazawa T, Niu DF, Mochizuki K, Kawasaki T, Yamane T, Katoh R (2010) Hypoxia-inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Hum Pathol 41(11):1550–1557
Ceruti S, Franceschi C, Barbieri D, Malorni W, Camurri A, Giammarioli AM, Ambrosini A, Racagni G, Cattabeni F, Abbracchio MP (2000) Apoptosis induced by 2-chloro-adenosine and 2-chloro-2′-deoxy-adenosine in a human astrocytoma cell line: differential mechanisms and possible clinical relevance. J Neurosci Res 60:388–400
Minelli A, Bellezza I, Tucci A, Rambotti MG, Conte C, Culig Z (2009) Differential involvement of reactive oxygen species and nucleoside transporters in cytotoxicity induced by two adenosine analogues in human prostate cancer cells. Prostate 69:538–547
Mirabet M, Mallol J, Lluis C, Franco R (1997) Dipropylcyclopentylxanthine triggers apoptosis in Jurkat T cells by a receptor-independent mechanism. Cell Death Differ 4:639–646
Morello S, Petrella A, Festa M, Popolo A, Monaco M, Vuttariello E, Chiappetta G, Parente L, Pinto A (2008) Cl-IB-MECA inhibits human thyroid cancer cell proliferation independently of A3 adenosine receptor activation. Cancer Biol Ther 7:278–284
Boyum A (1968) Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl 97:7
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Ohta A, Sitkovsky M (2011) Methylxanthines, inflammation, and cancer: fundamental mechanisms. Handb Exp Pharmacol 200:469–481
Himer L, Csoka B, Selmeczy Z, Koscso B, Pocza T, Pacher P, Nemeth ZH, Deitch EA, Vizi ES, Cronstein BN, Hasko G (2010) Adenosine A2A receptor activation protects CD4+ T lymphocytes against activation-induced cell death. FASEB J 24(8):2631–2640
Mills JH, Kim DG, Krenz A, Chen JF, Bynoe MS (2012) A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J Immunol 188(11):5713–5722
Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, Fortini C, Leung E, Mac Lennan S, Borea PA (2004) Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation. Mol Pharmacol 65(3):711–719
Sitkovsky MV, Ohta A (2005) The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol 26:299–304
Panjehpour M, Karami-Tehrani F (2004) An adenosine analog (IB-MECA) inhibits anchorage-dependent cell growth of various human breast cancer cell lines. Int J Biochem Cell Biol 36:1502–1509
Feng Y, Wu J, Feng X, Tao D, Hu J, Qin J, Li X, Xiao W, Gardner K, Judge SI, Li QQ, Gong J (2007) Timing of apoptosis onset depends on cell cycle progression in peripheral blood lymphocytes and lymphocytic leukemia cells. Oncol Rep 17:1437–1444
Müller CE, Ferre S (2007) Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Recent Pat CNS Drug Discov 2(1):1–21
Müller CE, Jacobson KA (2011) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta 1808(5):1290–1308
Shelton JR, Cutler CE, Oliveira M, Balzarini J, Peterson MA (2012) Synthesis, SAR, and preliminary mechanistic evaluation of novel antiproliferative N 6,5′-bis-ureido- and 5′-carbamoyl-N 6-ureidoadenosine derivatives. Bioorg Med Chem 20(2):1008–1019
Böhm L, Roos WP, Serafin AM (2003) Inhibition of DNA repair by Pentoxifylline and related methylxanthine derivatives. Toxicology 193(1–2):153–160
Garuti L, Roberti M, Bottegoni G (2009) Small molecule aurora kinases inhibitors. Curr Med Chem 16(16):1949–1963
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) within the Graduiertenkolleg GRK 677 (S.K.L, C.E.M.) and the NRW-Forschungsschule Biotech Pharma (A.C.S., C.E.M.). We thank H. Eltzschig and S. Zug (University of Tübingen, Germany) and A. Bill (University of Bonn, Germany) for their support in real-time PCR experiments. The technical assistance of N. Florin is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Anke C. Schiedel and Svenja K. Lacher contributed equally.
Rights and permissions
About this article
Cite this article
Schiedel, A.C., Lacher, S.K., Linnemann, C. et al. Antiproliferative effects of selective adenosine receptor agonists and antagonists on human lymphocytes: evidence for receptor-independent mechanisms. Purinergic Signalling 9, 351–365 (2013). https://doi.org/10.1007/s11302-013-9354-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-013-9354-7