Abstract
Purinergic signalling is involved in a number of physiological and pathophysiological activities in the lower urinary tract. In the bladder of laboratory animals there is parasympathetic excitatory cotransmission with the purinergic and cholinergic components being approximately equal, acting via P2X1 and muscarinic receptors, respectively. Purinergic mechanosensory transduction occurs where ATP, released from urothelial cells during distension of bladder and ureter, acts on P2X3 and P2X2/3 receptors on suburothelial sensory nerves to initiate the voiding reflex, via low threshold fibres, and nociception, via high threshold fibres. In human bladder the purinergic component of parasympathetic cotransmission is less than 3 %, but in pathological conditions, such as interstitial cystitis, obstructed and neuropathic bladder, the purinergic component is increased to 40 %. Other pathological conditions of the bladder have been shown to involve purinoceptor-mediated activities, including multiple sclerosis, ischaemia, diabetes, cancer and bacterial infections. In the ureter, P2X7 receptors have been implicated in inflammation and fibrosis. Purinergic therapeutic strategies are being explored that hopefully will be developed and bring benefit and relief to many patients with urinary tract disorders.
Similar content being viewed by others
Synopsis
Introduction
Urinary Bladder
-
Innervation of bladder
Parasympathetic cotransmission
Sympathetic cotransmission
Intramural bladder neurones and pelvic ganglia
Neuromodulation in the bladder
Central control of bladder function
Afferent pathways in bladder
Nociception and purinergic mechanosensory transduction
Evidence for ATP involvement in the micturition reflex
-
Smooth muscle
P2X receptors mediating contraction of the bladder
P2Y receptors mediating relaxation of the bladder
P1 receptors mediating relaxation and contraction of the bladder
Extracellular calcium, calcium channel blockers and potassium channel openers
Involvement of prostaglandins in purinergic signalling
Ectoenzymatic breakdown of ATP
-
Urothelium, suburothelial myofibroblasts and umbrella cells
-
Perinatal development and ageing of purinergic signalling in urinary bladder
Perinatal development
Ageing
-
Plasticity of purinergic signalling in bladder
Changes occurring during pregnancy or hormone therapies
Changes due to selective denervation
Bladder grafts
Hibernation
-
Purinergic signalling in the human bladder in health and disease
Healthy bladder
Overactive bladder syndrome
Detrusor overactivity
Neurogenic detrusor overactivity
Idiopathic detrusor overactivity
Bladder pain syndrome/interstitial cystitis (BPS/IC)
Bladder outflow obstruction
Botulinum toxin and ATP release
Multiple sclerosis
Post-irradiation bladder dysfunction
Ischaemic bladder
Chronic alcohol consumption and bladder function
Vitamin E deficiency
Diabetes
Bladder Cancer
Benign prostatic hyperplasia
Enterocytoplasty bladders
Bacterial infection
Urethra
Ureter
-
Functional expression of purinoceptors
-
Renal colic
-
P2X7 receptors and ureteral inflammation and interstitial fibrosis
Concluding comments
Urinary bladder
Innervation of bladder
Parasympathetic cotransmission
Atropine-resistant responses of the urinary bladder to stimulation of parasympathetic nerves were recognised for many years ([147, 290, 406, 686]; see [106]) and were later shown to be due to non-cholinergic, non-adrenergic transmission [19, 384, 463]. However, it was not until 1972 that evidence was presented to support the view that the atropine-resistant component in guinea-pig bladder was purinergic, i.e., due to adenosine 5′-triphosphate (ATP) released from the parasympathetic nerves supplying the bladder [117]. The evidence in this paper included: mimicry of the non-adrenergic, non-cholinergic (NANC) nerve-mediated excitatory responses by ATP (Fig. 1a); block of contractions both to NANC nerve stimulation and to exogenous application of ATP, but not to acetylcholine (ACh), by quinidine; and depression of NANC responses during tachyphylaxis produced by high concentrations of ATP. Direct evidence for ATP release from NANC nerves came in later papers [113] (Fig. 1b). Later studies have offered unequivocal support for this hypothesis (see [106]), not only in guinea-pig bladder [83, 112, 254, 287, 314, 325, 356, 482, 556, 721], but also the bladders of many other species, including: mouse [4, 301, 682, 714]; pig [253]; hamster [561]; marmoset and ferret [500]; dog [653]; monkey [166]; cat [417, 664]; shrew [312]; sheep [162, 166]; rat [53, 80, 93, 289, 326, 545, 680]; rabbit [134, 211, 254, 310, 321, 420, 442, 763] and human [74, 313, 333, 540, 589, 695, 725]. The prejunctional inhibition of both cholinergic and purinergic components of the nerve-mediated responses of the rat bladder by adenosine was taken as evidence in support of cotransmission ([545]; see also [499]).
a Contractile responses of the guinea-pig bladder strip to intramural nerve stimulation (NS; 2 Hz, 0.2 ms pulse duration, supramaximal voltage for 20 s) and ATP (8.5 μM). Atropine (1.4 μM) and guanethidine (3.4 μM) were present throughout. b Effect of changing the Ca2+ concentration on the release of ATP from the guinea-pig isolated bladder strip during stimulation of intramural nerves. Upper trace: mechanical recording of changes in tension (g) during intramural NS (2 Hz, 0.2 ms pulse duration, supramaximal voltage for 20 s). Lower trace: concentration of ATP in consecutive 20-s fractions of the superfusate. The Ca2+ concentration in the superfusate varied as follows: (i) 2.5 mM (normal Krebs); (ii) 0.5 mM; (iii) 0.25 mM; (iv) 2.5 mM. The successive contractions were separated by 60-min intervals as indicated by the breaks in the mechanical trace. Atropine (1.4 μM) and guanethidine (3.4 μM) were present throughout. The temperature of the superfusate was between 22 °C and 23 °C. (a and b Reproduced from [113], with permission from Elsevier.) c The effect of α,β-methylene ATP (α,β-meATP) on the response of isolated guinea-pig bladder strips to NS, ATP (∆) and histamine (Hist). Upper trace: control responses; lower trace, desensitization attained by five successive applications of α,β-meATP (50 μM, filled triangle), at 4-min intervals, completely abolished nerve-mediated and ATP induced contractions, although histamine-induced contraction is only slightly reduced. (Reproduced from [356], with permission from Elsevier.) d Rabbit urinary bladder detrusor, sucrose-gap recording at 33 °C, in the presence of atropine 0.3 μM. Effect of α,β-methylene ATP (α,β-meATP) on excitatory junction potentials (EJPS) evoked by field stimulation (filled circle, 0.5 Hz, 0.3 ms, 5 V, continuously) before (left trace) and during desensitization with α,β-meATP (10 μM) (right trace). At control membrane potential, EJPS are no longer visible during desensitization with α,β-meATP. (Reproduced from [310], with permission from Elsevier.) e Fluorescent histochemical localization of quinacrine in whole-mount stretch preparation of adult rabbit urinary bladder showing a ganglion cell containing at least six fluorescent nerve cells. The nuclei (arrow) are non-fluorescent. Calibration bars = 50 μm. (Reproduced from [172], with permission from Elsevier)
Following the initial proposa1 that ATP contributed to the contractile responses of the urinary bladder to parasympathetic nerve stimulation [97], much debate followed, as indeed it did about the general concept of purinergic neurotransmission. Ambache et al. [18] published a paper entitled "Evidence against purinergic motor transmission in guinea-pig bladder" based mainly on the relative insensitivity of the bladder to ATP and the inability of ATP to match precisely the atropine-resistant neurogenic responses, extending their earlier conclusion [19], although earlier papers had pointed out the close mimicry of the responses of ATP to atropine-resistant responses in terms of onset and decline [94, 473]. However, at that time the rapid ectoenzymatic breakdown of released ATP was not clearly recognised and the desensitisation of responses to ATP was not taken into account. Weetman and Turner [717] also argued against purinergic transmission on the basis of the lack of specific effects of several ATP receptor blocking agents that had been claimed to be effective on ATP responses in the guinea-pig taenia coli: “quinidine reduced the response to nerve stimulation without affecting the histamine controls, although this was probably due to local anaesthetic effect” and “phentolamine and two experimental drugs (2,2′-prydiylisatogen, and 2,2′-methoxyphenylisatogen), that are active against ATP-induced relaxation of the guinea-pig isolated taenia, were non-specific in their blockade of contractions of the bladder to nerve stimulation”. The lack of effect of theophylline or dipyridamole on the excitatory junction potentials (EJPs) in the rabbit bladder in response to intramuscular nerve stimulation was also taken as evidence against ATP being the non-cholinergic excitatory transmitter [167], but since these agents only affect the P1 receptor-mediated actions of adenosine, but not ATP, this was clearly not a valid argument. Tetrodotoxin-resistant release of ATP was taken to indicate ATP release from muscle during transmural stimulation and argued against ATP as a neurotransmitter in the rabbit bladder [138]. Since responses to electrical field stimulation in the presence of atropine were reduced, but not abolished, following desensitisation of the ATP receptor, it was concluded that ATP was unlikely to be the sole non-cholinergic motor transmitter in the rat detrusor [445].
Despite these reservations, several other laboratories confirmed and extended the evidence in favour of purinergic transmission. Dean and Downie [211] showed that desensitisation with ATP selectively depressed responses to ATP and to field stimulation (particularly at low frequencies), but not those in response to carbachol. Burnstock et al. [113] extended their earlier findings: quinacrine, a fluorescent dye know to bind to high levels of ATP in granular vesicles, produced positive staining in neurons and nerve fibres in the bladder; release of ATP during stimulation of NANC excitatory nerves was demonstrated using the firefly luciferin–luciferase assay method (also reported in [112]); and sympathectomy with 6-hydroxydopamine did not affect the release of ATP in response to intramural nerve stimulation. Compared to ATP, 100-fold lower concentrations of the slowly degradable analogue β,γ-methylene ATP (β,γ-meATP) were shown to mimic contractions of the atropine-resistant responses of the rat bladder, suggesting that the relative insensitivity of the bladder to ATP is due to its rapid degradation to adenosine 5′-monophosphate (AMP) and adenosine, which cause relaxation of the bladder [93]. The functional effects of purinergic innervation of the rabbit urinary bladder were also reported [420].
Evidence for purinergic and cholinergic components of the responses of the bladder to parasympathetic nerve stimulation in an in vivo preparation of urethane-anaesthetised guinea-pigs has been presented [556]. In anaesthetised cats, the ganglion stimulants, nicotine and dimethyl-phenylpiperazinium, increased intravesicular pressure by an atropine-resistant mechanism which was mimicked by ATP [381]. In a later study of the in vivo responses of the cat bladder to pelvic nerve stimulation it was concluded that purinergic transmission plays a role in the initiation of bladder contraction and perhaps in the initiation of urine flow, in contrast to cholinergic transmission that is involved in maintenance of contractile activity and flow [668]. In a recent study, evidence was presented that the purinergic component of parasympathetic cotransmission mediated Ca2+ signals that provide the initial Ca2+/calmodulin activation of myosin light chain kinase in smooth muscle, while the muscarinic receptors provide supporting sustained responses [682]. Purinergic neurotransmission was impaired in myosin Va-deficient mouse bladders indicating that myosin Va plays a major role in the vesicular ATP transport from varicosities [170].
In the late 1970s and the l980s, neuropeptides, particularly vasoactive intestinal peptide (VIP), became the favoured contenders for NANC transmission in a variety of preparations, including those of the lower urinary tract and penile erectile tissues (see [23, 311]), but in a study designed to compare the effects of substance P (SP),VIP and its structurally related polypeptide peptide histidine isoleucine, on the guinea-pig bladder with the affects of field stimulation and ATP [454], the slow sustained excitation elicited by VIP contrasted clearly with the fast transitory responses elicited by both ATP and field nerve stimulation. In a later study, Meldrum and Burnstock [482] showed that P2 purinergic receptor desensitisation with α,β-methylene ATP (α,β-meATP) did not alter the responses to VIP while blocking NANC excitation. Copper inhibits purinergic transmission in the bladder and the copper(i) chelater, neocuproine, enhances bladder activity by facilitating purinergic excitatory responses [268].
Release of β-nicotinamide adenine dinucleotide (β-NAD) has been reported during electrical field stimulation of intrinsic nerves in the human bladder [90]. The release is unaffected by guanethidine, but increased by capsaicin, suggesting that sensory nerves might be the origin of the release of β-NAD, rather than sympathetic or parasympathetic nerves.
Various compounds that inhibit ATP-induced contractions also inhibit the responses induced by electric field stimulation [106, 617]. NANC nerve-mediated responses of strips of guinea-pig urinary bladder were markedly reduced following desensitisation with ATP, but only slightly with guanosine 5′-triphosphate (GTP) or cytidine 5′-triphosphate (CTP) [449]. Reactive blue 2 was reported to antagonise selectively the ATP-induced relaxations of the guinea-pig distal colon [364]. Reactive blue 2 was also shown to inhibit the responses to ATP and to NANC nerve stimulation in both guinea-pig and rat bladders [150]. At about this time, arylazido-aminopropionyl ATP (ANAPP3) was also proposed as a specific antagonist to ATP [298] and was shown to inhibit contractile responses of the cat and guinea-pig bladder to both ATP and pelvic or intramural nerve stimulation [52, 664, 721]. In the rabbit bladder ANAPP3 blocked the atropine-resistant neurogenic response, but apparently not responses to exogenous ATP [442]. Kasakov and Burnstock [356] showed that the slowly degradable analogue of ATP, α,β-meATP, produced selective desensitisation of the P2 purinoceptor and that it abolished NANC excitatory responses of the guinea-pig urinary bladder (Fig. 1c). This was confirmed in later studies of both guinea-pig and rat bladder [83, 401]. In the first study of mouse bladder, α,β-meATP was shown to abolish the response to ATP and greatly reduce the NANC component of the neurogenic response [4]. At about the same time, α,β-meATP desensitisation experiments also supported NANC excitatory transmission in the bladders of ferret and marmoset [500].
After suramin was shown to be a reversible P2 purinergic receptor antagonist in the mouse vas deferens [217], it was reported to reduce the responses to both purinergic agonists and the NANC component of neural responses in the guinea-pig, rat and shrew bladders [312, 314, 680]. In a study of the effects of suramin on the responses to nerve stimulation and ATP in the bladder muscle strips from guinea-pigs, rabbits, monkeys and sheep and detrusor strips from humans, it was show that it produced parallel inhibition in guinea-pig and rabbit, but in sheep and human tissue, where the purinergic nerve component was smaller, the effect of suramin was difficult to assess because of increase in spontaneous activity [166].
Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) was introduced as a P2X antagonist in the vas deferens in 1992 [405] and later was also shown to be effective in selectively antagonising P2X purinoceptor-mediated contractions in the rabbit urinary bladder produced by exogenous α,β-meATP and by purinergic nerve stimulation [680, 763]. P2X receptors in the guinea-pig bladder were shown to be more sensitive to PPADS than suramin, and diadenosine tetraphosphate (Ap4A) appeared to be acting through this P2X receptor since, like ATP responses, responses to Ap4A were abolished alter desensitisation with α,β-meATP [687].
Reactive blue 2 reduced the post-contractile relaxation of the bladder neck of the male mini-pig and this was taken to suggest that P2Y purinoceptors were involved [678].
EJPs elicited by stimulation of sympathetic nerves supplying the guinea-pig vas deferens were first recorded by Burnstock and Holman in the early 1960s [119, 120], although it was not until the 1980s that EJPs were shown to be due to the actions of neuronally released ATP [627]. The first recordings of EJPs (using both microelectrode and sucrose-gap methods) in smooth muscle cells of the urinary bladder in response to intramural nerve stimulation were published in 1983 [167], although the authors presented data that they interpreted as not supporting the proposal that ATP was the NANC-excitatory transmitter. Later papers, however, clearly showed that the atropine-resistant EJPs recorded in the bladders of rabbits, guinea-pigs and pigs were inhibited by desensitisation of the ATP receptor with α,β-meATP (Fig. 1d) and were therefore the result of purinergic transmission [82, 254, 310]. In other studies EJPs recorded in the guinea-pig bladder were reduced by the ATP antagonist suramin [95,166].
In an elegant study employing the whole-cell patch clamp technique on single smooth muscle cells isolated from guinea-pig bladder, it was possible to show that ATP could closely mimic the EJP and this was taken as support for the concept that ATP is the transmitter responsible for fast neurotransmission in the bladder [332]. Patch-clamp studies on isolated smooth muscle cells from sheep bladder suggested that Cibacron blue is a potent activator of a Ca2+-dependent outward current in addition to its action as a purinergic antagonist [162]. Using a voltage-clamp of smooth muscle cells from guinea-pig bladder, ATP, adenosine 5′-diphosphate (ADP), α,β-meATP and β,γ-meATP were shown to produce rises in fast inward transmembrane current, while GTP, inosine 5′-triphosphate (ITP), AMP and adenosine failed to activate this current [465].
Analysis of the EJPs recorded in the guinea-pig bladder [88] showed first that they varied greatly in both amplitude and time course even when recorded from cells at similar distances from the stimulating electrodes, and second that, as the strength of field stimulation was reduced, the amplitude of EJPs was decreased in two or three discrete steps, rather than gradually. Spontaneous EJPs (sEJPs) were also recorded from most cells. The authors raised the possibility that EJPs result from the activation of two different membrane conductances and that the variation in EJP amplitude may be related to the degree of coupling between smooth muscle cells in and between muscle bundles. EJPs, but not sEJPs, recorded in mouse bladder, were abolished by tetrodotoxin, but both EJPs and sEJPs were abolished by NF279, a P2X1 receptor antagonist [602]. The authors also showed that phorbol dibutyrate potentiated EJP amplitudes, but not those of sEJPs, probably by increasing ATP release from the nerve varicosities.
Frequent ATP-mediated spontaneous depolarisations (probably sEJPs) were recorded in mouse detrusor muscle and their frequency and whole cell Ca2+ flashes increased in the absence of the urothelium, suggesting that an inhibiting agent released from the urothelium may modulate the spontaneous activity of the bladder [485]. Spontaneous depolarisations or sEJPs were abolished by NF449, a P2X1 receptor antagonist [746].
The concept of cotransmission is now well accepted (see [98, 108]), including strong evidence that ATP acts as a cotransmitter with noradrenaline (NA) in the sympathetic nervous system (sea [101, 103]). It is surprising that there is much less information about cotransmission with ATP in the parasympathetic nervous system. ATP is released from synaptic vesicles from motor nerve terminals together with ACh in the rat diaphragm and in teleost electric organs [612, 767], and there is also evidence that ATP is coreleased with ACh from sympathetic nerves supplying catfish chromatophores [256]. The paucity of information about parasympathetic cotransmission may partly be due to the fact that it is easier to eliminate surgically postganglionic sympathetic nerves, or chemically denervate with sympatholytics such as guanethidine or 6-hydroxydopamine, than it is to disrupt surgically or chemically postganglionic parasympathetic nerves.
Perhaps the first hint that ATP and ACh might be cotransmitters in parasympathetic nerves supplying the bladder came from an ultrastructural study of nerves supplying the smooth muscle of the bladder, where the vesicular composition of nerve profiles containing small agranular and large opaque vesicles led the authors to propose that cholinergic and NANC transmitters were colocalised [308]. Further indirect evidence for purinergic cotransmission came from binding studies and regional studies of the responses of strips taken from five different areas of the rabbit bladder, where it was shown that the bladder body and base showed parallel sensitivity to urecholine (a muscarinic agonist), and to ATP. Other indirect evidence for purinergic cotransmission came from studies of purified botulinum neurotoxin (BTX) type A and neuromuscular transmission in the guinea-pig bladder; both cholinergic and purinergic components of the excitatory responses to nerve stimulation were significantly reduced by BTX [455].
It seems likely from pharmacological studies of bladder contractility that a spectrum of nerves exist, utilising different proportions of ATP and ACh, from predominantly ATP in cat and guinea-pig through to roughly 50:50 in rat and dog to predominantly ACh in healthy human bladder.
The M3 muscarinic receptor appears to be the subtype primarily responsible for excitatory cholinergic transmission in the bladder, although M2 receptors may also be involved in some species [221, 222]. Functional impairments found in M3 knockout (KO) mice were milder than those elicited by active blockade of muscarinic receptors in wild type (WT) mice, suggesting that non-cholinergic (purinergic) transmission can compensate for the chronic loss of M3 receptors [328].
In a whole rabbit bladder in vitro preparation exogenous ATP and electrical field stimulation in the presence of atropine produced a transient rapid rise in intravesical pressure [423]. However, these purinergic responses did not result in significant bladder emptying, suggesting that they may be complementary, but functionally different, from those which occur in response to cholinergic transmission [415, 420]. A comparison of the purinergic responses in the whole bladder in vitro preparations of cat and rabbit revealed both qualitative and quantitative species differences [417]. In particular, the component of purinergic NANC transmission in the bladder of the cat was considerably less than that found for the rabbit bladder. Studies of whole rabbit bladders by another group led to the conclusion that neurally released ATP is important in the initiation of micturition, but ACh is necessary for bladder emptying [134]. Another study, in which substances were administered intra-arterially to the whole rabbit bladder preparation, showed that pre-treatment with isoprenaline, a β-adrenergic agonist, significantly inhibited contractions to ACh or ATP [426]. Thus in pathological conditions such as bladder–urethral dyssynergia, involving simultaneous firing of sympathetic and parasympathetic nerves, both cholinergic and purinergic bladder contractions could be suppressed while the urethra was contracted.
Pharmacological studies of the cat bladder in vivo [206, 238, 419] showed a clear atropine-resistant contraction evoked by pelvic nerve stimulation, which had a purinergic component [664]. Purinergic transmission also contributes to bladder contractions evoked by stimulation of the hypogastric (sympathetic) nerves [665].
In another study using unanaesthetised rats and continuous cystometry, it was shown that ATP, or α,β-meATP, administered intra-arterially close to the bladder, produced rapid, phasic and dose-dependent increases in bladder pressure and micturition immediately after injection [326]; pre-treatment with α,β-meATP blocked the effects of ATP. Carbachol produced sustained increases in bladder pressure and micturition. After blockade of the micturition reflex with morphine (10 μg intrathecally), ATP, α,β-meATP and carbachol were unable to induce bladder emptying. In a later study, this group examined purinergic responses in unanaesthetised rats with bladder outlet obstruction [327]. In an in vivo anaesthetised rat model, it was shown that the contractile response of the bladder to pelvic nerve stimulation consists of a phasic purinergic component that predominates at lower stimulation frequencies, followed by a tonic cholinergic component predominating at higher frequencies [530].
Evidence has been presented for purinergic transmission in the urinary bladder of pithed rats [289]. Spinal electrical stimulation (L6–S2) evoked increases in intravesicular pressure and the major NANC component was antagonised by α,β-meATP or PPADS. ATP produced dose-dependent increases in intravesicular pressure. It was concluded that purinergic transmission mediated by ATP acting on P2X receptors represents a major component of excitatory innervation of the rat urinary bladder.
Implantation of chronic bladder catheters and cystometrography was used to study the micturition reflexes in unanaesthetised rats and it was shown that, at the spinal level, xanthine-sensitive P1 (adenosine) receptors, probably located on an excitatory interneuronal link, inhibited the volume-evoked micturition reflex [630].
In urethane-anaesthetised rats, intrathecal administration of α,β-meATP or adenosine-5′-(γ-thio)-triphosphate (ATPγS) induced transient bladder contractions followed by a prolonged depression of reflex bladder activity recorded under isovolumetric conditions [370]. These agents also elicited bladder contractions and a secondary inhibition of reflex bladder contractions when administered intravenously. The excitatory affect of intravenous α,β-meATP was reduced by atropine or hexamethonium, a ganglionic blocking agent, indicating that this response was mediated in part by stimulation of the parasympathetic pathways to the bladder and in part by a direct effect on the bladder smooth muscle. On the other hand, the excitatory effect of intravenous ATPγS was not suppressed by atropine but was completely blocked by hexamethonium, indicating that the effect was mediated by reflex activation of non-cholinergic pathways to the bladder, possibly due to stimulation of bladder afferent nerves. It was concluded that excitatory and inhibitory purinergic mechanisms are present not only in the peripheral nervous system/smooth muscle of the lower urinary tract but also in reflex pathways in the spinal cord that control micturition.
Prolonged modulation of the parasympathetic micturition reflex was studied in anaesthetised cats, reflex discharges being recorded from a thin pelvic nerve branch to the bladder and evoked by stimulation of the remaining ipsilateral bladder pelvic nerves or urethral branches of the pudendal nerve [344]. The results have led to the proposal that prolonged modulation of the micturition reflex represents physiological adaptive processes preserving bladder function.
Experiments in the anaesthetised rat [577] have evaluated the effects on bladder function of local injection of ATP or α,β-meATP into the brain. Injection of either agent into the periaqueductal grey matter or the locus coeruleus, two brainstem areas which play an important role in the supraspinal control of micturition, led to increases in pelvic neural activity and bladder pressure and/or rate of bladder contractions. Since electrical stimulation in these same areas also activates the parasympathetic pathways to the bladder [529], it seems likely that purinergic excitatory receptors are present in the micturition reflex circuitry in the brain.
Sympathetic cotransmission
Sympathetic innervation of the detrusor has in general been reported to be sparse, although the trigone region is relatively densely innervated by sympathetic nerves (see [16, 311]). Sympathetic nerve fibres reach the bladder largely in the hypogastric nerve. Stimulation of the hypogastric nerve may cause an increase or decrease in pressure in the urinary bladder, but always excites the urethra (see [165, 207, 330]). Physiologically inhibitory sympathetic transmission in the detrusor is important during the filling phase of the voiding cycle [209, 402, 461]. In addition to transmitter released from sympathetic nerves acting directly on smooth muscle, they may act prejunctionally on parasympathetic nerve terminals to inhibit both cholinergic and non-cholinergic (purinergic) excitation, which are invoked in the voiding phase of the micturition cycle.
Although purinergic transmission predominantly originates from postganglionic parasympathetic or intramural nerves, in the cat at least, ATP may also be released from the hypogastric nerve. This nerve is predominantly sympathetic, but may also contain parasympathetic elements (see [430]). When the hypogastric nerve is stimulated in the cat it causes the bladder to contract; this contraction is reduced by ANAPP3 [663, 664, 670], implying that ATP is being released. Furthermore, 6-hydroxydopamine, which destroys sympathetic nerves, prevents this contractile response, indicating that the ATP is released from sympathetic nerves [665]. Guanethidine, in a dose which blocked the bladder relaxation induced by hypogastric nerve stimulation and mediated by NA acting on β-adrenergic receptors [205], did not affect hypogastric nerve-mediated excitation [665]. However, in guanethidine-treated animals, ANAPP3 blocked the excitation. These findings suggest that ATP may be released from the hypogastric nerve.
Nicotinic-induced contractions of the guinea-pig bladder in the presence of atropine, were abolished by desensitisation of the P2X receptor with α,β-meATP [297]. Several possible mechanisms might be involved, namely, that nicotine might produce a contraction by activating nicotinic receptors on:
-
1.
Parasympathetic nerve terminals coreleasing ACh and ATP
-
2.
Sympathetic nerve terminals coreleasing NA and ATP
-
3.
Intramural bladder neurones that corelease ATP with peptides
One of the basic features of neuromuscular cotransmission appears to be that the cotransmitters released act synergistically (see [100]). There is evidence that NA and ATP released as cotransmitters from sympathetic nerves act synergistically [299], but there do not appear to be any reports of synergistic cotransmission in the urinary bladder involving either parasympathetic (ACh and ATP) or sympathetic (NA and ATP) nerves. It is also likely that ATP is a cotransmitter with NA in perivascular sympathetic nerves supplying blood vessels in the bladder (see [101]).
Intramural bladder neurones and pelvic ganglia
Intramural ganglia have been described in the bladder of several mammalian species, including humans [15, 111, 173–175, 266, 566]. Quinacrine, a fluorescent dye, that selectively labels high levels of ATP bound to peptides in granular vesicles, stained a subpopulation of neurons in ganglia in the guinea-pig bladder [112, 176] (Fig. 1e). Subpopulations of neurones in bladder ganglia also stained positively for VIP, somatostatin, SP, 5-hydroxytryptamine and acetylcholinesterase. Thus, intramural ganglia, perhaps largely parasympathetic postganglionic neurones, contain and probably release ATP. They also respond to microapplication of ATP [111]. No intramural neurones have been observed in the rat bladder, but several weeks after unilateral pelvic ganglion destruction, intramural neurones were consistently observed along the remnants of nerves in the originally denervated half of the bladder [690].
Parasympathetic ganglia on the surface of the cat urinary bladder have provided useful preparations for examining synaptic modulatory mechanisms (see [199]). These ganglia contain several types of principal ganglion cells (coexpressing various neuropeptides, ACh, NA, ATP and nitric oxide [NO]) as well as small intensely fluorescent cells. They receive an innervation from both parasympathetic and sympathetic preganglionic axons. Parasympathetic preganglionic axons, which arise in the sacral segments of the spinal cord and travel in the pelvic nerve, represent the principal excitatory pathway to the cholinergic–purinergic ganglion cells [194, 200], which in turn provide an excitatory input to the detrusor smooth muscle. The sympathetic innervation originates in the thoracolumbar (TL) spinal cord and passes to the bladder via the hypogastric nerves and the sympathetic chain. The sympathetic system exerts an inhibitory control over activity of the detrusor muscle and an excitatory input to the trigone and urethra.
Various purinergic agonists including ATP, α,β-meATP, ADP, AMP, adenosine and 2-chloroadenosine (2-ClADO) administered intra-arterially depress cholinergic transmission and depress the bladder contractions elicited by stimulation of preganglionic axons in the pelvic nerve [198, 669]. High doses of ATP also produce postganglionic firing in unstimulated, decentralised ganglia, indicating a direct excitatory effect of ATP on bladder ganglion cells. Other nucleotides and related substances such as cyclic AMP (cAMP), dibutyryl cAMP, adenosine, inosine and ITP have weak or no effects on transmission. ATP, ADP, AMP and adenosine are equipotent in depressing transmission, whereas 2-ClADO, an agent that is more resistant to cellular uptake and metabolism, is ten times more potent than adenosine. This indicates that metabolism could have a significant influence on the effectiveness of purinergic agents. This is also indicated by the effect of dipyridamole to enhance and prolong the inhibitory responses to injected purinergic agents. Dipyridamole, which slows the cellular uptake of adenosine, enhances the inhibitory actions of AMP and adenosine as well as those of ATP and ADP, suggesting that the latter agents can be converted to adenosine.
Theophylline and caffeine block the inhibitory effects of purinergic agents on ganglionic transmission and on neurally evoked bladder contractions, indicating that the inhibition is mediated by P1 receptors. The P1 receptors appear to be located presynaptically as well as postsynaptically on the ganglion cells. Since the sympathetic input has a modulatory effect on transmission in bladder ganglia [205, 399, 400], and since ATP can be released as a cotransmitter from sympathetic nerve terminals, sympathetic nerves may be a source of ATP released within the bladder ganglion, although the principal sympathetic modulatory mechanisms in the ganglia are mediated by NA acting on α-adrenoceptors [361].
Since ATP can be released from adrenergic and cholinergic nerves, studies were conducted on cat bladder ganglia to determine whether endogenously released substances might elicit purinergic inhibition. Extracellular recordings in situ did not detect a theophylline-sensitive (purinergic) component in either the inhibition of ganglionic transmission elicited by stimulation of sympathetic nerves (hypogastric) or the heterosynaptic inhibition elicited by stimulation of preganglionic axons in the pelvic nerves [202]. On the other hand, intracellular recordings from isolated bladder ganglia in vitro identified a non-cholinergic, slow hyperpolarising synaptic potential elicited by high intensity and high frequency (40 Hz) stimulation of the preganglionic nerve trunk [11, 609]. The non-cholinergic slow hyperpolarisation, which has amplitude of approximately 5 mV and duration of 30s, is increased in amplitude and duration by dipyridamole, an agent which blocks the uptake of adenosine, and is reduced in amplitude by adenosine deaminase, an enzyme that metabolises adenosine. Caffeine, a P1 receptor antagonist, also blocks the synaptic potential. The slow hyperpolarising synaptic potential is mimicked by the administration of exogenous purinergic agonists (500 nM–1 mM), the relative order of potency being: 2ClADO >> AMP > adenosine > ADP > ATP. This order of potency is consistent with a response mediated by a P1 receptor.
While hyperpolarising responses are detected in virtually all bladder ganglion cells (92 %), a smaller percentage of cells (52 %) exhibit fast depolarising responses to ATP and other purinergic agonists [609]. In some cells the fast depolarising response is followed by a more prolonged slow hyperpolarisation lasting 1–1.5 min. The ATP depolarisation is associated with a decrease in membrane resistance, reverses polarity at −7 mV and is dependent on the concentration of Na+ and not K+ ions. The relative order of potency among purinergic agents to produce the fast depolarisation is: ATP > ADP >> AMP > adenosine. This depolarising action of ATP no doubt mediates the ganglionic excitatory effects of ATP noted during in situ experiments [669].
The precise physiological roles of purinergic agents in the control of transmission in bladder ganglia need resolution. The demonstration of purinergic slow hyperpolarising potentials following stimulation of preganglionic nerves indicates that purinergic agents can be released in ganglia during neural activity. However, since ATP is present in bladder postganglionic neurones as well as in cholinergic and adrenergic nerve terminals, there are various possible sources of purinergic transmitter. In addition, although adenosine has been proposed as the inhibitory transmitter [11], it is possible that the extracellular catabolism of ATP to adenosine could be important in the mediation of the slow hyperpolarising responses. Whether this catabolism could occur within the latency for evoking the hyperpolarising potentials is not known. It is also important to note that preganglionic nerves contain cholinergic and adrenergic postganglionic axons as well as preganglionic axons and therefore various neural pathways could be involved in eliciting the slow hyperpolarising potential. Adenosine deaminase has been identified in sacral preganglionic neurones in the rat [604]. This has prompted the speculation that preganglionic pathways may be purinergic as well as cholinergic. It is not known whether a similar situation exists in the cat.
Single electrode voltage-clamp techniques in rabbit vesical parasympathetic ganglion cells [528] showed that ATP and ADP, but not AMP or adenosine, caused an inward current associated with increased conductance. Suramin and Reactive blue 2, but not hexamethonium reversibly depressed the actions of ATP and ADP, suggesting that ATP activates cation channels through P2X receptors in rabbit parasympathetic neurones. Application of ATP also modulates the amplitude of nicotinic fast excitatory postsynaptic potential in the rabbit vesical parasympathetic ganglia [527].
P2X receptors have been demonstrated in pelvic ganglion neurones of rat [762] and guinea-pig [761]. In the rat, evidence from the pharmacological characteristics of pelvic ganglion neurones in response to P2 agonists and antagonists recorded with the whole cell voltage-clamp technique combined with in situ hybridisation and immunohistochemistry led to the conclusion that P2X2 receptors are the predominant P2X subtype present in about 39 % of the neurones. In contrast, in the guinea-pig, at least three distinct P2X receptors were shown to be present in different subpopulations of neurones in the pelvic ganglion, probably P2X2 and P2X3 homomultimers in 5 % and 70 % of the neurones, respectively, and about 25 % with heteromultimeric P2X2/3 receptor, but the possibility that an unidentified P2X receptor subtype is also present was not discounted.
Neuromodulation in the bladder
ATP released as a cotransmitter at various sites including sympathetic, parasympathetic, sensory and motor nerve terminals, at synapses in autonomic ganglia and in the central nervous system (CNS) can be broken down by ectoenzymes to adenosine that then acts on prejunctional P1 receptors to modulate the release of neurotransmitters (see [6, 180, 208, 251, 358, 453, 479, 575, 629, 632, 705]). P1 receptors on the nerve terminals on the bladder are of the A1 subtype, while postjunctional smooth muscle receptors are of the A2 subtype [6, 100]. At some sites, especially where the junctional cleft is narrow, ATP itself acts on prejunctional P2 receptors to modulate transmitter release [252, 610, 706, 723]. This has also been described in rat bladder [372]. ATP can also act as a postjunctional modulator of cholinergic (nicotinic) transmission to skeletal muscle [291] and of sympathetic responses in vas deferens [299].
In the rat urinary bladder, ATP, adenosine and α,β-meATP were all shown to produce a dose-dependent and reversible inhibition of the atropine-resistant contractile responses to transmural nerve stimulation [183], suggesting that both P1 and P2 receptors are present on terminals of parasympathetic nerves in this bladder preparation. A study of natural products from Nauclea latifolia, a tree that grows in the northern part of Nigeria, showed that the leaf extract was very potent in potentiating purinergic neurotransmission and ATP-induced contractions in rat bladder, while the root extract depressed purinergic contraction by a direct action on smooth muscle, since it did not modify ATP-induced contractions [685].
In the mouse bladder, 5-hydroxytryplamine (5-HT), perhaps released from circulating platelets, was shown to potentiate strongly the predominantly purinergic parasympathetic nerve mediated responses, probably via 5-HT1B receptors [301]. This was later confirmed in guinea-pig bladder, where the effects of 5-HT were shown to be mediated by 5-HT2A and 5-HT4 receptors [486] and also in bladder of pigs [123], humans [161] and rabbits [44]. In contrast, γ-aminobutyric acid (GABA), acting through GABAB prejunctional receptors, inhibited nerve-mediated contractions in mouse bladder [596]. Morphine, however, did not alter the responses to nerve stimulation or exogenously applied ATP in the mouse bladder. Cannabinoid CB1 receptors reduced nerve-mediated contractions in some, but not all mammalian species, but did not affect responses to ACh or α,β-meATP, providing evidence for prejunctional modulation of release of transmitter from parasympathetic nerves [467, 555]. Dopamine reduced the twitch (purinergic) component of the nerve-mediated response of the rat bladder via prejunctional D2 receptors [230], while the contractile response of the rat urinary bladder is mediated by muscarinic M3 receptors, M2 receptors have a modulatory action on purine-evoked relaxations [264].
In the rat bladder, bradykinin and SP have been shown to facilitate the purinergic component of parasympathetic nerve responses and the responses to exogenous ATP, implying that in this case the mechanism of action is at the postjunctional site [7, 552]. Neuropeptide Y (NPY) also potentiated α,β-meATP contractions in the rat bladder, but not ACh-evoked contractions, suggesting that NPY, which is present in sympathetic and parasympathetic pathways to the rat detrusor [360], contributes to transmission in two ways: (1) by promoting non-cholinergic motor transmission and (2) by inhibiting prejunctionally cholinergic transmission [336, 681, 770]. Endothelin-1 produced long lasting potentiation of both NANC and ATP responses in rat bladder [215].
In the guinea-pig bladder, histamine potentiated the responses to ATP and NANC nerve stimulation, suggesting an action at postjunctional sites. However, it did not potentiate the response to ACh or cholinergic component of the nerve-mediated response [551].
The mechanisms underlying stimulation of bladder contractions by the selective neurokinin NK2 receptor agonist [β-Ala8]NKA(4–10) were examined in the anaesthetised guinea-pig [8]. Pretreatment of the animals with both atropine and α,β-meATP or by ganglion blockers led to complete blockade of neurokinin NK2 receptor-induced contractions. These results suggest that stimulation of NK2 receptors located on capsaicin-sensitive sensory nerves (where NK2 receptors have been demonstrated autoradiographically) leads to bladder contractions via both cholinergic and purinergic parasympathetic motor nerves. SP and bradykinin both potentiate the neurogenic responses of the guinea-pig bladder by influencing the purinergic component of the excitatory motor innervation, apparently at a postjunctional site [552]. Clenbuterol, a β2-adrenoceptor agonist significantly inhibited the contractile response to both nerve stimulation and ATP by a postjunctional action [319].
Central control of bladder function
Activation of P2 receptors in both periaqueductal grey matter and Barrington's nucleus/locus coeruleus regions of the rat brain stem is implicated in the neural mechanisms that generate patterns of activity in the parasympathetic innervation of the urinary bladder ([577]; see also [107]).
Afferent pathways in bladder
Reviews concerned with afferent signalling in the lower urinary tract are available [185, 197, 210, 656].
Most of the afferent supply of the bladder and urethra originates in dorsal root ganglia (DRG) at the lumbosacral (LS) region of the spinal cord and passes peripherally through the pelvic nerve [195, 514]. Although smaller in number, afferents also project to the urogenital tract through sympathetic nerves (hypogastric) from DRG at the TL level (see [340, 497, 741]). In addition, the afferent supply of the external urethral sphincter travels by the pudendal nerve to the sacral region of the spinal cord (see [315]).
A study was carried out to explore differences in sensitivity to purinergic agonists in LS and TL DRG sensory neurons that innervate the bladder via hypogastric and pelvic nerves, respectively. It was shown that the majority of LS neurons (93 %) were sensitive to α,β-meATP compared to 50 % of TL neurons [189]. The authors concluded that bladder pelvic and hypogastric splanchnic afferents are functionally distinct and likely to mediate different sensations arising from the urinary bladder. The central projections of pelvic and pudendal afferents overlap within the spinal cord allowing integration of somatic and parasympathetic motor activity [201].
Afferent signals originating in the bladder have been shown to be regulated via presynaptic P2X3 and P2X2/3 receptors in the spinal cord to facilitate the sensory input of the micturition reflex [350]. Activity of afferents from one region of the pelvic viscera can influence the efferent output to another region. Thus, stimulation of anal, rectal or vaginal afferents can inhibit micturition [160]. It is also now becoming clear that sensory afferents can modulate efferent activity in peripheral autonomic ganglia as well as in the CNS. Afferent neurones can release neurotransmitters from peripheral terminals in the viscera as well as from central terminals in the spinal cord. In the bladder, transmitter release from sensory nerve endings may also play an efferent role by direct postjunctional effects on the detrusor muscle [196, 457].
The roles of ATP released from urothelial cells and suburothelial myofibroblasts on various bladder functions have been considered at length in several reviews [32, 411, 643, 730] and evidence presented that urothelial-released ATP may alter afferent nerve excitability [55,197]. The kinetics of ATP release from bladder urothelium has been described [425]. Topical activation of a cannabinoid agonist acting via the CB1 receptor leads to a decrease in the firing activity evoked by urinary bladder distension, mainly on high threshold afferents [708]. The authors present evidence to suggest that the cannabinoid agonist acts directly on sensory afferents and/or an indirect action by interacting with the purinergic system involving urothelial cells.
Mechanosensitive afferents of the rat bladder include A-δ, capsaicin-sensitive and capsaicin-insensitive C-fibres; the activities of primary afferents produced by intravesical introduction of ATP are mediated mainly through a subset of capsaicin-sensitive C-fibres [9]. Decreased intravesical stretch-evoked ATP release was found in isolated bladders from transient receptor potential vanilloid (TRPV) 4 KO mice, suggesting a role for TRPV4 in urothelium-mediated transduction and in voiding behaviour [259]. Pressure-stimulated ATP release from the bladder urothelium is blocked by inhibitors of vesicular transport, connexin hemichannels and ATP-binding cassette (ABC) protein family transporters [713]. Eviprostat, a phytotherapeutic drug for benign prostatic hypoplasia, also inhibits pathological bladder activity by decreasing ATP release from urothelium [641]. Differential purinergic signalling in LS and TL DRG mediated by P2X2, P2X3 and P2X2/3 receptors has been reported in both naïve and bladder-inflamed mice and rats [140].
Nociception and purinergic mechanosensory transduction
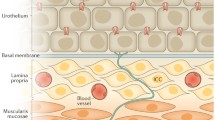
Burnstock [104] put forward a hypothesis, indicating that distension of epithelial cells lining the tubes (including ureter) and sacs (including urinary bladder) in the body leads to release of ATP which then acts on P2X3 receptors on suburothelial sensory nerves to modulate afferent firing that can lead to bladder voiding reflexes and pain (see Fig. 2a).
a Schematic of hypothesis for purinergic mechanosensory transduction in tubes (e.g., ureter, vagina, salivary and bile duct, gut) and sacs (e.g., urinary and gall bladders, and lung). It is proposed that distension leads to release of ATP from epithelium lining the tube or sac, which then acts on P2X2/3 receptors on subepithelial sensory nerves to convey sensory/nociceptive signals to the CNS. (Reproduced from [104], with permission from Blackwell Publishing.) b P2X3 receptor immunoreactivity in the mouse bladder. Immunostaining is seen on small suburoepithelial nerve fibres. Calibration bar = 50 μm. (Reproduced from [158], with permission from Nature Publishing Group)
Early evidence for ATP release from rabbit urinary bladder epithelial cells by hydrostatic pressure changes was presented by Ferguson et al. [237], who speculated about this being the basis of a sensory mechanism. Prolonged exposure to a desensitising concentration of α,β-meATP significantly reduced the activity of mechanosensitive pelvic nerve afferents in an in vitro model of rat urinary bladder [513]. Later, it was shown that mice lacking the P2X3 receptor exhibited reduced inflammatory pain and marked urinary bladder hyporeflexia with reduced voiding frequency and increased voiding volume, suggesting that P2X3 receptors are involved in mechanosensory transduction underlying both inflammatory pain and hyperreflexia and a role in physiological voiding reflexes was also suggested [158]. A later study from this group, using P2X2 KO mice and P2X2/P2X3 double KO mice revealed a role for the P2X2 subtype too in mediating the sensory effect of ATP [157]. In a systematic study of purinergic mechanosensory transduction in the mouse urinary bladder, ATP was shown to be released from urothelial cells during distension and discharge initiated in pelvic sensory nerves was mimicked by ATP and α,β-meATP and attenuated by P2X3 antagonists as well as in P2X3 KO mice; P2X3 receptors were localised on suburothelial sensory nerve fibres (Fig. 2b) [704]. Single unit analysis of sensory fibres in the mouse urinary bladder revealed both low and high threshold fibres sensitive to ATP contributing to physiological (non-nociceptive) and nociceptive mechanosensory transduction, respectively [580]. Several functionally distinct populations of bladder sensory nerves have been recognised, not all of which respond to ATP [753]. Purinergic agonists increase the excitability of afferent fibres to distension [580]. It appears that the bladder sensory DRG neurons, projecting via pelvic nerves, express predominantly P2X2/3 heteromultimer receptors [760]. BTXA, which has antinociceptive effects in treating interstitial cystitis (IC), inhibits distension-mediated urothelial release of ATP in conditions of bladder inflammation [624]. Clinically, there is a significantly increased level of ATP release from the urothelium isolated from bladder pain syndrome (BPS) patients during mechanical stimulation, compared to those from normal human bladders [397].
Evidence for ATP involvement in the micturition reflex
ATP given intravesically stimulates the micturition reflex in awake freely moving rats, probably by stimulating suburothelial C-fibres, although it was suggested that other mediators might also be involved [543]. Studies of resiniferatoxin desensitisation of capsaicin-sensitive afferents on detrusor over-activity induced by intravesical ATP in conscious rats, supported the view that increased extracellular ATP has a role in mechanosensory transduction and that ATP-induced facilitation of the micturition reflex is mediated, at least partly, by nerves other than capsaicin-sensitive afferents [10, 87]. ATP has also been shown to induce a dose-dependent hyperreflexia in conscious and anaesthetised mice, largely via capsaicin-sensitive C-fibres; these effects were dose-dependently inhibited by PPADS and 2′(3′)-O-(2,4,6-trinitrophenyl) ATP (TNP-ATP) [317]. P2X1 and P2X3 receptors play a fundamental role in the micturition reflex in female urethane-anaesthetised rats; P2X3 receptor blockade by phenol red raised the pressure and volume thresholds for the reflex, while P2X1 receptor blockade diminished motor activity associated with voiding [373]. These authors also suggested that P2Y1 receptor blockade may remove an accommodating inhibitory drive to rat detrusor muscle. Voiding dysfunction involves P2X3 receptors in conscious chronic spinal cord injured rats, which raises the possibility that P2X3 receptor antagonists might be useful for the treatment of neurogenic bladder dysfunction. Urinary bladder hyporeflexia was evident in P2X3 receptor KO mice [158]. Intravesical ATP stimulates overactivity and the micturition reflex in awake, freely moving rats [543]. The cholinergic and purinergic contributions to the micturition reflex were studied in conscious female rats with long-term bladder outlet obstruction [440, 590]. It was found that there was a substantial decrease in the detrusor muscle responses to M3 receptor-mediated contractility, while there were no significant changes in the contractile responses to α,β-meATP or electrical field stimulation. Activation of α1D-adrenoceptors in rat urothelium facilitates the micturition reflex, by increasing the release of ATP; naftopidil, an α1D-adrenoceptor antagonist, inhibited distension-induced bladder afferent activity and distension-evoked ATP release [337, 640]. Activation of urothelial TRPV4 and subsequent release of ATP trigger a novel neural mechanism that regulates the late phase of detrusor muscle contractility after micturition that occurs if the bladder is not empty [61].
Isolectin B4 (IB4) protein-labelled afferent fibres, most of which express P2X3 receptors, sprout after spinal cord transection at time points preceding the emergence of bladder overactivity; interruption of IB4 afferent sprouting by IB4-saporin treatment increased voiding efficiency [769]. Gentle mechanical stimulation of the skin can inhibit transmission of vesical afferent volleys into the vesico-vesical parasympathetic reflex pathways, leading to inhibition of rhythmic micturition contractions [303].
Activation of muscarinic receptors localised near the luminal surface of the bladder affects voiding functions via mechanisms involving ATP and NO release from the urothelium that in turn could alter the firing properties of afferent nerves, again supporting the view that urothelial-afferent nerve interactions can influence reflex voiding functions [393]. It has been suggested that blockade of TRPV1 receptors in urothelium with ERC-6211 results in a reduction in ATP release, leading to a decrease in bladder reflex activity [137]. Interactions between A2A receptors and dopaminergic receptors in the CNS have also been claimed to control the micturition reflex [376].
Smooth muscle
Since the potent actions of ATP in the bladder were first recognised, substantial advances have been made in identifying the ATP receptors involved. From cloning and second messenger studies in the early 1990s, it was proposed that receptors to ATP belong to two families: a P2X ion channel family and a P2Y G protein-coupled receptor family [1, 571]. Seven P2X subtypes and eight P2Y subtypes are currently recognised [107].
Although ATP undisputedly contracts the urinary bladder of most species (see [23, 94, 311]) it can also induce relaxation. Thus it is likely that multiple purinergic receptors are present in the bladder. A number of recent studies have been carried out to try to identify the receptor subtypes mediating excitation and inhibition in the lower urinary tract.
P2X receptors mediating contraction of the bladder
An analysis of the excitatory actions of purine and pyrimidine nucleotides on the guinea-pig bladder revealed the following order of potency: β,γ-meATP > ATP > GTP = CTP > ADP, while adenosine, AMP, guanosine diphosphate, guanosine, cytosine diphosphate, cytosine monophosphate and cytidine had no contractile activity up to 1 mM [450]. In retrospect, this potency series, although incomplete, was already suggestive of P2X receptor-mediated responses, particularly P2X1 or P2X3, especially since ATP responses exhibited rapid desensitisation, a property of the latter receptors [112]. In the rabbit urinary bladder, the response to ATP is biphasic; however following desensitisation by α,β-meATP, the response became monophasic, suggesting that more than one type of excitatory receptor to ATP was present [139].
Studies of the responses of the feline bladder to purines and pyrimidines revealed the following potency order: 5′-adenylimidodiphosphate = β,γ-meATP > ATPγS = 2-methythio ATP (2-MeSATP) > ATP > uridine 5′-triphoshate (UTP) = CTP = GTP [667]. Reactive blue 2 and Coomassie brilliant blue G, mistakenly regarded as selective P2Y receptor antagonists at that time, both antagonised the purine-induced contractions, prompting the suggestion that multiple purine receptors were present in detrusor smooth muscle [667]. In the rat and dog urinary bladder, a potency order of α,β-meATP > ATP > ADP was reported [650] and the authors concluded that three subtypes of purinoceptor might be present in rat bladder; Pl receptors (mediating relaxation), P2X receptors, and another type of P2 receptor (mediating contractions); but only a single receptor type (P2X) in dog bladder (Fig. 3a).
a Contractile effects of various purines on rat and dog urinary bladder smooth muscle strips. Contractions were induced by ATP (1 mM), ADP (1 mM), α,β-methylene ATP (α,β-meATP, 10 μM) and α,β-methylene ADP (α,β-meADP, 0.1 mM). In each panel, responses to ATP, ADP and α,β-meATP were obtained from the same strip, whereas those to α,β-meADP were obtained from a different strip. (Reproduced from [650], with permission from John Wiley and Sons.) b Transverse section of rat urinary bladder detrusor muscle immunostained for P2X1 receptors. Calibration bar = 100 μm. (Reproduced from [412], with permission from Elsevier)
Contractile responses of the rat bladder induced by ATP and α,β-meATP were fast and transient, reaching a maximum in about 20s; in contrast, contractions in response to adenosine 5′-O-2-thiodiphosphate (ADPβS) and UTP were slower and sustained and were barely affected by α,β-meATP desensitisation, suggesting two different receptor subtypes [77]. It has been proposed that about 20 % of the neurogenic contraction of rat bladder is mediated by purinergic receptors sensitive to ADPβS [284]. The diadenosine polyphosphate, Ap4A, contracts the guinea-pig bladder and since this was abolished after desensitisation with α,β-meATP and by suramin, PPADS and nifedipine, it is likely to act through P2X receptors [687].
An early study of the characteristics of [3H]ATP binding to homogenates of the rabbit urinary bladder using radioligand filtration methodology showed high affinity binding, favouring the view that ATP receptors are present in smooth muscle membranes [416].
Autoradiographic studies of the distribution of [3H]α,β-meATP in the rat bladder showed a high level of labelling in the smooth muscle of the detrusor, but none in the urethra, which correlated closely with the pharmacology [69]. In a parallel study, high- and low-affinity binding sites for [3H]α,β-meATP were demonstrated in rat urinary bladder membranes and displacement experiments with unlabelled purinoceptor ligands confirmed that [3H]α,β-meATP mainly binds to P2X receptors with a potency order of α,β-meATP > β,γ-meATP > suramin > ATP > ADP > 2-MeSATP >> adenosine [71]. In later studies, autoradiographic localisation and characterisation of [3H]α,β-meATP binding sites were described in the urinary bladders of guinea-pigs, rabbits, cats and humans [72, 74, 487, 758]. High-affinity binding sites for [35S]ATPγS in the human bladder have also been described [487]. cDNA encoding P2X purinoceptors from human bladder smooth muscle were expressed in oocytes, pharmacologically characterised [233] and subsequently shown to be the hP2X1 receptors.
Immunohistochemical studies which have been carried out with specific antibodies to the different P2X receptor subtypes showed that P2X1 receptors are the dominant subtype in the membranes of the smooth muscle cells in the rat detrusor and also vascular smooth muscle in blood vessels in the bladder [412] (Fig. 3b). In another immunohistochemical study of the rat bladder, clusters of P2X1 receptors were described on smooth muscle cells, some, but not all, of which were closely related to nerve varicosities [278]. In a later paper by this group, it was shown that P2X1 receptors migrated and formed clusters beneath parasympathetic nerve varicosities during development [220].
Northern blotting and in situ hybridisation have also shown the presence of P2X1 mRNA in urinary bladder [694]. Northern blot analysis also detected the expression of the human P2X4 receptor gene in the bladder [213], but immunohistochemistry has not detected staining for this receptor. In another study, the P2X1 receptor was shown to be the functional P2X receptor subtype mediating contraction of the mouse bladder, although some diffuse immunostaining was detected for P2X2, P2X4 and P2X7 receptors [702]. Briefly elevated calcium levels sensitise rat bladder smooth muscle purinergic P2X1 receptors to promote desensitisation recovery [181]. A recent paper has claimed that there is an interaction of P2X2 and nicotinic ACh receptors in smooth muscle cells from the base, but not the dome, of rat urinary bladder [342].
Interstitial cells of Cajal (ICCs) have been claimed to be present, not only in gut, where they express P2X and P2Y receptors [122, 696], but also in the bladder of guinea-pigs [193, 712], mice [476, 752] and humans [476, 608]. ICCs (also referred to as myofibroblasts; see [727]) are considered to be pacemaker cells, regulating smooth muscle activity. A comparison of electrical and mechanical activity and ICCs in urinary bladders from WT and the mouse W/W v mutation phenotype, showed upregulation of the purinergic component of contraction for the W/W v mice, perhaps involving ICCs [477]. In a recent paper, it was claimed that ICCs in mouse bladder express NTPDase2 [752], indicating specific mechanisms for ATP disposition.
The first nucleotide structure–activity relationship (SAR) studies on bladder were carried out by Noel Cusack and colleagues [115]. Neither 2-chloro-ATP nor 2-MeSATP was significantly more effective than ATP itself in producing contraction of guinea-pig bladder. Enantiomers showed some stereoselectivity at low concentrations, but this was lost or even reversed with higher concentrations. However, it was recognised that the rapid ectoenzymatic breakdown of ATP to adenosine, which relaxes the bladder [93], probably distorted the results. Adenylyl 5′-(β,γ-methylene)-diphosphonate (β,γ-meATP) had much greater contractile effects on the guinea-pig bladder than ATP and the enantiomer of β,γ-meATP, L-β,γ-meATP, was even more potent, probably because l-β,γ-meATP was completely resistant to degradation [178], although it was inactive on P2Y receptors in the taenia coli [306]. Of the phosphorothioate analogues of ATP, ADP and AMP, it was found that adenosine 5′-O-1-thiotriphosphate, ATPβS, Rp-ATPβS and Sp-ATPβS were much more potent than ATP in the bladder [116]. Among the 2-methylthio derivatives of β,γ-meATP the potency order in the bladder was: difluoromethylene > methylene > dichloromethylene [179]. None of the analogues were degraded by ectonucleotidases, and restoration of the electro-negativity of the triphosphate chain did not further enhance their potency. Unlike the effects of these agents on the taenia coli, the order of potency in the bladder did not reflect their order of acidity; this may be because some distortion of the triphosphate chain is necessary to accommodate the bulky chloro groups, whereas the difluoro analogue is sterically more similar to ATP [66]. A study of the SARs of nucleotide effects on excitatory P2 receptors in the bladder provided evidence that dephosphorylation of ATP analogues reduced pharmacological potency.
Using a radioligand binding assay it was found that adenosine, adenine and xanthine had no significant effect on [3H]α,β-meATP binding to membrane fractions prepared from rat urinary bladder, while pentasodium triphosphate and disodium pyrophosphate could effectively displace the binding; these results were taken to indicate that the phosphate side chain of ATP and its analogues is the key structure responsible for the binding to P2X receptors [73]. A further study of the affinities of ATP derivatives for P2X purinoceptors in rat bladder was carried out with modifications of the polyphosphate chain as well as the adenine and ribose moieties [75]. Replacement of the bridging oxygen in the triphosphate chain of ATP with a methylene or imido group markedly increased the affinity, modifications at N 6, N 1 and C-8 positions of the purine base reduced the affinity of ATP, attachment of an alkylthio group to the C-2 position increased affinities, while replacement of the 3′-hydroxyl group on the ribose with substituted amino or acylamino groups produced more potent P2X receptor agonists. Diadenosine polyphosphates (ApnA) were also shown to displace [3H]α,β-meATP binding with a rank order of potency Ap6A > Ap5A > Ap4A >> Ap3A >> Ap2A.
Suramin, PPADS and Reactive blue 2 competitively displaced the binding of [3H]α,β-meATP to P2X receptors. An extensive study of SARs for derivatives of ATP as agonists at P2X and P2Y receptors was carried out [118]. For example, 3′-benzylamino-3′-deoxy-ATP was found to be very potent in the guinea-pig bladder, but was inactive at P2Y receptors. There are reviews that discuss the developments of P2X receptor antagonists [240, 260, 651].
P2Y receptors mediating relaxation of the bladder
ATP, as well as adenosine, has been shown to reduce pelvic nerve-evoked bladder contractions; however, since methylxanthines did not fully antagonise the responses [669], this suggests that P2Y receptors as well as P1 (adenosine) receptors might be involved in purine inhibition. These P2Y receptors are likely to be on nerve terminals in the bladder providing prejunctional inhibition of release of excitatory neurotransmitters and both Reactive blue 2 and Coomassie brilliant blue G antagonise the inhibitory actions of ATP and analogues on nerve-mediated contractions [667]. However, these experiments did not exclude the possibility that ATP could also be acting through postjunctional P2Y receptors on bladder smooth muscle mediating direct relaxation, although usually masked by the dominating contractile actions of ATP through P2X receptors.
The first direct evidence for ATP-induced relaxation of smooth muscle came from studies of the mouse bladder [76]. In carbachol precontracted preparations, ATP elicited initial contractions, followed by a sustained relaxation, while on K+ precontracted preparations, ATP caused relaxation only, which was not inhibited by 8-phenyltheophylline (8-PT). The order of potency for relaxation was: 2-MeSATP > ATP > β,γ-meATP, perhaps indicative of P2Y receptors A biphasic response of bladder strips in response to ATP was also described in the rat [78]. The initial contraction was abolished after desensitisation of the P2X receptor with α,β-meATP, revealing a clear relaxation response to ATP. The evidence put forward that these relaxant responses were mediated by a P2Y receptor was first that 2-MeSATP was more potent than ATP, and second that G proteins were involved, since the G protein activator, guanosine 5′-O-3-thiotriphosphate, significantly potentiated the relaxant responses, while the G protein blocking agent, guanosine 5′-O-2-thiodiphosphate (GDPβS), completely abolished the relaxation; these agents had no effect on the ATP-induced contractions.
In the mini-pig bladder it appears that the neurally evoked relaxation which follows the initial cholinergic contraction of the bladder neck is mediated by P2Y receptors [680]. In another study of pig bladder neck, it was shown that ATP after breakdown to ADP caused relaxation via P2Y1 receptors and, after breakdown to adenosine, relaxation via A2A receptors [294]. 8-PT did not affect the relaxations, negating P1 receptor mediation, but the P2Y antagonist Reactive blue 2 reduced the relaxations by about 80 %. However, it is well known that Reactive blue 2 can produce non-specific inhibitory effects with prolonged exposure or with high concentrations, so more experiments will need to be carried out to confirm this claim. Nevertheless, supporting evidence for postjunctional purinoceptor subtypes has come from studies in the marmoset urinary bladder [481] where a biphasic response to ATP was demonstrated. The potency order for the relaxation phase was ATP = 2-MeSATP ≥ ADP >> α,β-meATP. When the initial contraction was abolished by desensitisation with α,β-meATP, a relaxation response clearly remained, which was abolished by the G protein inactivator, GDPβS. The relaxation was unaffected by 8-PT, or the NO synthase (NOS) inhibitor, N G-nitro-l-arginine (l-NOARG), but was blocked by Cibacron blue which is regarded as a P2Y antagonist, at least on native receptors, and which did not affect the contractile responses to ATP. Since N-tosyl-l-phenylalanine chloromethyl ketone, an inhibitor of cAMP-dependent protein kinase (PK) A, significantly shifted the curve for the ATP-induced relaxation to the right, it was suggested that the subtype of the P2Y G protein-coupled receptor involved might be one that acts through adenylate cyclase.
Evidence for P2Y1 receptors in the rat bladder, which have been claimed to mediate relaxation, has also been provided using reverse transcription-polymerase chain reaction (RT-PCR), Northern blotting and in situ hybridisation [531]. The in situ hybridisation technique showed the presence of P2Y1 mRNA in detrusor smooth muscle and blood vessels in the bladder, but no positive staining was seen in urethral smooth muscle. P2Y receptors might also mediate excitatory responses in the bladder because UTP contracts the rat bladder [78]. According to current thinking UTP would be acting through a P2Y2 or P2Y4 receptor or may be acting through an as yet unidentified P2Y receptor subtype. It has been claimed [515] that, in addition to a P2X receptor (probably the P2X1 subtype), there are ADPβS-sensitive receptors in the rat bladder. Since these receptors mainly depend on Ca2+ release from intracellular stores and this is mediated by the production of inositol triphosphate via the activation of phospholipase C, it seems likely that the ADPβS-sensitive receptor might be a P2Y receptor.
In summary, the evidence for the presence of P2Y receptors on smooth muscle of the bladder is growing and the concept that purinergic innervation may play a role at the start of micturition by inducing the initial detrusor muscle contraction and at the same time relaxing the bladder neck, is attractive. In a more recent paper, it was concluded that in the rat bladder contractions are mediated predominantly via P2X1 receptors, while P2Y2, P2Y4, P2Y6 and A2B receptors mediate relaxation [37]. In addition, ATP has been reported to activate pro-inflammatory responses through P2Y receptors expressed by a human uroepithelial cell line infected by bacteria [599] and a recent paper has shown using RT-PCR the expression of P2Y1, P2Y2 and P2Y4 receptors in the human bladder [607], but only the P2Y4 receptor was found to be functional.
P1 receptors mediating relaxation and contraction of the bladder
In the initial study of the possible roles of purines in NANC transmission in the guinea-pig bladder [117], it was clearly shown that, in contrast to ATP and ADP, adenosine and AMP caused relaxation. In contrast to the contractions produced in the detrusor, ATP produced relaxation of the bladder neck [295]. However, since ATP is rapidly broken down to adenosine, and ATP and adenosine are equipotent in this tissue, it seems likely that ATP exerts its action via a P1 receptor. In support of this view, α,β-meATP, which is slowly degradable, is without effect on the bladder neck and both ATP and adenosine concentration curves are shifted to the right by the P1 receptor antagonist 8-PT. However, 8-PT failed to modify nerve-mediated relaxations in the bladder neck [295, 377], so it was interesting that the NANC responses were later identified as nitrergic [554, 674].
In the rat bladder, adenosine and 5′-N-ethylcarboxamidoadenosine (NECA) inhibited the contractions induced by carbachol. Since NECA was much more potent than cyclopentyl adenosine and adenosine, the P1 receptor subtype involved was likely to be A2 [521].
It has been claimed that P1(Al) receptors mediate 2-ClADO contractions in cat detrusor muscle and that the contraction depends on a pertussis toxin-sensitive Gi3 protein, phospholipase C-β3 and the release of intracellular Ca2+ [733]. There is loss of adenosine A2B receptor-mediated relaxation of the aged female rat bladder [539].
Extracellular calcium, calcium channel blockers and potassium channel openers
The sources of calcium for ATP contraction of the bladder smooth muscle have been examined. In the rat bladder the responses to ATP were reversibly abolished in Ca2+-free media and were never inhibited less than 45 % by verapamil and diltiazem. It was concluded that, while extracellular Ca2+ was largely involved in the actions of ATP, some intracellular Ca2+ was also involved [54, 318]. In the mouse bladder, responses to ATP and electrical field stimulation were also mainly dependent upon extracellular calcium [5]. In studies of guinea-pig detrusor and dispersed smooth muscle cells from rabbit bladder, it was concluded that stimulation of purinergic receptors opens an ion channel and allows influx of Ca2+, while muscarinic receptor stimulation mobilises intracellular Ca2+ via hydrolysis of inositol phospholipids [168, 325]. Experiments on cultured smooth muscle cells from rabbit bladder, performed with the fura-2 technique to measure changes in intracellular Ca2+, showed that ATP produced a rapid but transient increase in [Ca2+]i, while ACh produced a delayed, prolonged increase [534]. Studies on isolated smooth muscles cells from guinea-pig bladder, using a whole-cell voltage-clamp technique, confirmed that purinergic receptor stimulation opens non-selective cation channels, while muscarinic stimulation triggers Ca2+ release from intracellular stores [512].
Different types of Ca2+ channels are present prejunctionally and postjunctionally in the urinary bladder. L-type channels appear to be predominantly present in bladder smooth muscle [374, 466]. Stimulation of purinoceptors activates both Ca2+ influx through L-type calcium channels and Ca2+ release from intracellular Ca2+ stores [285]. Whereas ATP release from parasympathetic nerves in the bladder involves predominantly P- and Q-type calcium channels, ACh release depends primarily on N-type channels [714]. However, activation of P- and Q-type Ca2+ channels, though phosphorylation by PKC may be involved in the enhancing effect of the PKC activator, β-phorbol-12,13-dibutyrate, on the muscle contractions elicited by excitatory purinergic neurotransmission in mouse detrusor strips [429, 437].
Since ATP was known to act by opening Ca2+ channels, the effect of several calcium channel blockers on NANC nerve-mediated responses of the urinary bladder were examined. Early studies using rabbit and rat urinary bladder, hinted that, while terodiline was largely anticholinergic, nifedipine, an L-type Ca2+ channel blocker, might be effective against the NANC-mediated component [130, 320, 335, 459, 460]. Nifedipine (0.1–0.2 mg/kg) reduced the ATP-mediated contractions of guinea-pig bladder in vivo by 34–100 % [618]. Nifedipine was shown nearly to abolish the responses of the rat bladder to β,γ-meATP and the purinergic neuronal component, while a substantial proportion of the responses to ACh and the cholinergic neuronal component were resistant to nifedipine [70, 754]. Verapamil was more potent than diltiazem in inhibiting both ATP and NANC fast initial neurogenic responses, but ACh-mediated responses were also affected [53, 759]. Bay K 8644, a 1,4-dihydropyridine, which is an L-type channel activator, substantially increased the contraction of the bladder to β,γ-meATP and NANC nerve-mediated responses [70]. In another study of the rat bladder [456], the effects of nifedipine and Bay K 8644 were confirmed and ω-conotoxin, an N-type channel blocker, was shown to reduce the purinergic component of nerve-mediated responses, although to a lesser extent than those of the cholinergic component, and had little effect on the responses to either ATP or ACh. In the guinea-pig bladder, too, ATP-evoked contractions were markedly inhibited by dihydropyridine-like Ca2+ antagonists, such as nifedipine and nitrendipine, but not by D-600, ω-conotoxin or tetramethrin [359]. Benzodiazepine (diazepam) also antagonises the responses to ATP, probably by decreasing Ca2+ entry [600].
The potassium channel opener,YM934, was show to inhibit markedly the contractile responses of the guinea-pig detrusor smooth muscle to exogenously applied α,β-meATP, but only slightly inhibited the contractions produced by carbachol; it was concluded that YM934 may hyperpolarise the smooth muscle membranes by opening ATP-sensitive potassium channels and as a result may functionally inhibit the contractile response to purinergic nerve stimulation that elicits membrane depolarisation [470]. Other K+ channel openers cromakalim and ZM244085 were shown to hyperpolarise and reduce contractions of bladder smooth muscle; this effect was blocked by glibenclamide, but was unaffected by apamin [255, 428]. In a subsequent study, cromakalim was shown to affect profoundly the responses to exogenous ATP, but had little action on the responses to carbachol [80]. The authors concluded that cromakalim acts on purinergic transmission predominantly postjunctionally, whereas its minor action on cholinergic transmission is mainly at the prejunctional level.
A transgenic mouse containing the Ca2+-sensing molecule, G-cAMP, has been developed and has been used to show Ca2+ flashes in response to intrinsic nerve and ATP stimulation via P2 receptors [343].
Low concentrations of artificial sweeteners increased the maximum detrusor muscle contraction to α,β-meATP, but not to carbachol, suggesting that they act via modulation of L-type Ca2+ channels [191]. Two distinct types of Ca2+ signals have been identified in urinary bladder smooth muscle cells, using confocal microscopy combined with fast Ca2+ imaging techniques: global Ca2+ flashes and novel, smaller, localised purinergic Ca2+ transients. The global Ca2+ flashes represent Ca2+ influx during action potentials. The smaller, localised purinergic Ca2+ transients are not ryanodine receptor-mediated Ca2+ sparks, but instead represent Ca2+ influx through P2X receptors located on the muscle cells. These localised purinergic Ca2+ transients may represent the initial, crucial steps in the nerve-evoked cascade of events that leads to increases in intracellular Ca2+ and contraction of bladder smooth muscle by ATP released from nerve varicosities [292].
Involvement of prostaglandins in purinergic signalling
It was shown in 1974 that adenine nucleotides induce prostaglandin (PG) synthesis [517] and, soon after, evidence was presented that PGs were responsible for the rebound contractions of the guinea-pig taenia coli that follow stimulation of purinergic inhibitory nerves [114]. Since then, evidence has accumulated that PGs are generated in bladder smooth muscle as a result of purinergic neurotransmitter activity. In the guinea-pig, rabbit and monkey isolated detrusor, PGE2 and PGF2α caused potent contractions [345, 346]. In response to electrical field stimulation, there is an initial phasic contraction followed by a secondary tonic contraction. Indomethacin, a PG synthesis inhibitor, reduced the initial phasic contraction (purinergic) and the response to ATP [151, 212, 346]. PGE2 was later shown to be released from detrusor smooth muscle as a result of neural activity, but not from nerves [14], and atropine-resistant contractions were also reduced by the PG antagonist SC19220 [216].
ATP, but not adenosine, ACh or carbachol, evoked the release of PGs from detrusor muscle [22, 357]. In the rabbit detrusor muscle, ATP evoked a biphasic contraction consisting of an initial phasic contraction followed by a delayed secondary tonic contraction. Indomethacin prevented the secondary but not the initial phasic response [28, 322]. ADP produced only a slow tonic contraction that was almost abolished by indomethacin. Similar results were found in the human bladder [323]. The structural conformation of the polyphosphate chain of the ATP molecule is critical for stimulation of PG biosynthesis [92]. ATP can also lead to production of prostanoids in uroepithelial cells of the bladder, an affect which is enhanced in pathological conditions [565]. PGE2 production was significantly increased in the guinea-pig bladder lamina propria by 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate acting via both P2X and P2Y receptors [523].
Ectoenzymatic breakdown of ATP
It has been recognised for a long time that ectoenzymes can hydrolyse ATP released from nerves and non-neuronal cells. Much is now known about the various enzymes involved and the roles of the breakdown products in different tissues (see [768]). Burnstock [99] proposed the concept that ATP acts on P2 receptors and that, after breakdown by ectoenzymes to adenosine, it then acts via another receptor, the P1 receptor.
Studies of the guinea-pig bladder revealed that ATP analogues which are resistant to enzymatic degradation were more potent in eliciting a contraction via P2X receptors [718], although genuine differences in SARs do exist for methylene analogues of ATP that are only slowly degraded by ectonucleotidases [179].
The role of enzymatic degradation was also evaluated by studying the effect of putative inhibitors of ectonucleotidases on the pharmacological response of ATP in guinea-pig bladder [305]. Some inhibitors of ecto-ATPase were identified, including suramin and ethacrynic acid, which were strongly effective in enhancing the response to ATP; difluoro-dinitrobenzene was less effective, while N-ethylmaleimide, ATPγS and Reactive blue 2 were without effect. In a later study, ARL 67 l56 was identified as a potent ATPase inhibitor [164] which potentiated the response to ATP (but not α,β-meATP) and responses to atropine-resistant responses to nerve stimulation [722] (Fig. 4a and b). Cyclopiazonic acid, an inhibitor of sarcoplasmic ATPase, potentiated the contractile response of the guinea-pig bladder to exogenous ATP and NANC excitatory nerve stimulation but this was non-specific [764]. In the same paper, ecto-ATPase in the bladder was estimated to have a V max of 0.98 nmol P i 30 min−1 mg−1 wet tissue, with a K m of 881 μM ATP, respectively; cyclopiazonic acid (10 μM) inhibited ecto-ATPase activity by about 18 %. Subsequent studies [766] revealed that some divalent cations, such as Cu2+, Ni2+, Zn2+ and La3+, inhibit the ecto-ATPase activity in a concentration-dependent manner in the guinea-pig bladder, but not all of them potentiate contractions to ATP.
The effect of ARL 67156 in guinea-pig bladder strips on a contractions to exogenous ATP (100 μM, n = 16) and α,β-methylene ATP (α,β-meATP, 5 μM, n = 7) and b neurogenic contractions (1–8 Hz, for 20 s, n = 8). Open bars represent control responses and the hatched bars those in the presence of ARL 67156 (100 μM). **P < 0.01, ***P < 0.001. (a and b Reproduced from [722], with permission from Elsevier.) c Contractile responses of isolated strips of urinary bladder of adult and neonate (2- to 6-day-old) rabbits to nerve stimulation. Note that responses to nerve stimulation were also greater in neonatal tissue. Bars represent the mean response ± SEM for 5–7 experiments. N.S. significant difference, *P < 0.05 (Student's t-test for unpaired data). (Reproduced from [628], with permission from Elsevier)
Magnesium-dependent adenosine triphosphatase (Mg2+-ATPase) as well as 5′-nucleotidase and alkaline phosphatase were identified in the epithelial cells of the rat urinary bladder [756], perhaps functioning to degrade the ATP released from uroepithelial cells [237, 288, 379] during purinergic mechanosensory transduction [104, 513]. A recent study using RT-PCR has shown that eight members of the ectonucleoside triphosphate diphosphohydrolase (NTPD) family as well as 5′-nucleotidase are expressed in mouse bladder [750]. 5′-Nucleotidase was present exclusively in detrusor smooth muscle together with NTPD1, suggesting a mechanism for providing adenosine to act on P1 receptors on myocytes.
Urothelium, suburothelial myofibroblasts and umbrella cells
P2X3 receptors were found to be localised on the urothelium of both rat and human bladders [231]. ATP is released from the bladder urothelium in response to distension [237, 704], probably by vesicular exocytosis [510]. ACh and NO are also released from urothelial cells (see [58]). A report claims that the urothelium is the primary source of released ATP and NO in the rat urinary bladder, rather than nerves [505].
Extracellular Ca2+ regulates the stimulus-elicited ATP release from mouse urothelium [471] and rise in [Ca2+]i may control ATP release [726]. Distension-induced ATP release from mouse urothelium is regulated by the adenylyl cyclase–cAMP pathway [472]. It was suggested that this pathway might be involved in facilitating the micturition reflex or that it might cause an excess of ATP release in pathological conditions causing frequent urination. Release of ATP in response to stretch was higher in porcine urothelial and myofibroblast cell cultures compared to smooth muscle [144]. Cultured rat bladder urothelial cells release ATP when exposed to sustained hydrostatic pressure in the physiological threshold range that would trigger micturition (10–15 cm H2O) [535].
It has been claimed that pannexin-1 channels mediate P2X7 receptor-induced release of ATP from rat bladder mucosa, probably from both urothelial cells and myofibroblasts [672]. Cyclooxygenase inhibitors suppress ATP release from rat bladder epithelium via decreasing PGE2 action via prostanoid EP1 and/or EP3 receptors [660]. Both the vanilloid and acid-sensing ion channel (ASIC) systems contribute to acid-induced ATP release from urothelial cells [586]. Stretch and acid, but not capsaicin, are effective stimuli for ATP release from the porcine bladder mucosa [585].
The capsaicin-gated ion channel, TRPV1 is expressed by urothelial cells, as well as by suburothelial afferent nerve terminals and stretch-evoked ATP release was diminished in TRPV1 KO mice [62]. These findings indicate that TRPV1 receptors participate in normal bladder function and are essential for mechanically-evoked purinergic signalling by the urothelium. However, it was claimed in a recent paper that the mouse urothelial cell response to ATP is mediated by P2X, but not TRPV1 receptors [274].
Urothelium exerts inhibitory control over purinergic contractility produced by ATP or Ap4A [367]. In healthy cats, P2X1-7 and P2Y1,2,4 receptor subtypes were expressed throughout the bladder epithelium [63] and P2Y2, and to a lesser extent P2Y4, receptors in the urothelium of rats [153]. Knockdown of P2Y2 receptor expression using small interfering RNA resulted in reduction of ATP-evoked rise in [Ca2+]i in both human and rat bladder urothelial cells [636]. It has been reported recently that uridine diphosphate (UDP), via P2Y6 receptors, increases voiding frequency in humans via release of ATP from the urothelium and autocrine reinforcement of its release [616]. Urothelial cells can be activated by mechanical stretch and by ATP, ACh and other agents to release ATP as well as ACh, NO, PG, SP and nerve growth factor (NGF) (see [33, 59]).
Adenosine exerts negative feedback control of ATP release from the urothelium via A1 receptors [219]. In contrast stimulation of α3 nicotinic ACh receptors on bladder epithelium increases [Ca2+]i through extracellular influx and increases basal ATP release leading to increase in reflex bladder activity [51]. Cultured rat urothelial cells express functional M1, M2 and M3 muscarinic receptor subtypes, whose activation leads to ATP release, probably through mechanisms involving [Ca2+]i increases [392]. Muscarinic receptor agonists increased the release of ATP from urothelial cells in response to stretch and muscarinic receptor antagonists reduced the ATP release [726, 745]. The inhibitory effects of the anti-muscarinic agents, propiverine and imidefenacin, on bladder activity may be partly due to blocking an increase of ATP release from the urothelium [526]. BTXA prevents release of ATP from the urothelium and attenuates bladder afferent nerve firing [186]. Application of a TRPV4 agonist led to increased [Ca2+]i and released ATP [10]. Pituitary adenylate-cyclase activity peptide released ATP in rat bladder urothelium, thereby regulating micturition pathways [267]. Urothelial cells express multiple TRP and ASIC channels, whose activation produces ionic currents and Ca2+ influx. It is suggested that these properties may underlie the basic mechanisms for the release of transmitters, including ATP, NO and ACh that can affect afferent nerve signalling [394]. Spontaneous contractile activity of the urothelium was increased by α,β-meATP [498], suggesting mediation via P2X1 and/or P2X3 receptors. It has been suggested that urothelial purinergic receptors are important modulators of LS dorsal spinal neuronal activity and that enhanced LS neuronal signals result from activation of urothelial P2 receptors and C-fibres during noxious intravesical pressure stimulation [508].
Suburothelial myofibroblasts, which lie between urothelial cells and sensory nerve terminals, have been isolated from both human and guinea-pig bladder and shown to elicit ATP-generated transients via P2Y2 (and/or possibly P2Y4) receptors [727]. It has been suggested that these cells may be involved in an intermediate regulatory step in the sensation of bladder fullness between urothelial ATP release and afferent excitation [642]. In a later study it was shown that the predominant P2 receptor subtype in guinea-pig suburothelial myofibroblasts is the P2Y6 receptor, although there was weak expression of P2X3, P2Y2 and P2Y4 receptors [643]. Electrophysiological studies of suburothelial cells isolated from the human bladder showed that they were electrically active, responsive to ATP and perhaps coupled to neighbouring cells via gap junctions [642]. The urothelium and ATP suppressed carbachol-induced contractions of rat detrusor smooth muscle to a similar extent and this was interpreted to suggest an inhibitory role for ATP released from urothelium to modulate detrusor smooth muscle activity [597].
Specialised umbrella cells line the mucosal surface of the bladder and form a barrier between urine and the underlying tissue and bladder filling increases the apical surface area of these cells [32]. These cells are mechanosensitive. It is suggested that ATP released from the urothelium acts on P2 receptors on the umbrella cells to stimulate membrane insertion of the apical pole of the cells [713]. KO mice lacking expression of P2X2 and/or P2X3 receptors failed to show increases in apical surface area when exposed to hydrostatic pressure. A recent review discusses urothelial signalling in both physiology and pathophysiology, including the role of purines and pyrimidines [57].
Perinatal development and ageing of purinergic signalling in urinary bladder
Perinatal development
ATP and ACh are cotransmitters in parasympathetic nerves supplying the adult bladder. In an early study of the responses of the rabbit urinary bladder to autonomic neurotransmitters during development, receptors to ATP and ACh were recognised in the newborn animals, while adrenoceptors were poorly expressed at this stage [418]. In a later study, newborn rabbit bladders were shown to generate much greater tension in response to ATP than in adult tissue and then decline, while the response to cholinergic agonists did not decline [362, 628, 755] (Fig. 4c). Crowe and Burnstock [172] carried out a histochemical study using markers for cholinergic, adrenergic and purinergic transmission during perinatal development of rabbit bladder. Acetylcholinesterase-positive nerve fibres and ganglion cells and quinacrine-positive ganglion cells were both present on day 23 of gestation, while quinacrine-positive varicose nerve fibres were first seen on day 24. At foetal day 26, large numbers of ganglia (25–38), each containing 30–40 quinacrine-positive neurones, were seen in the detrusor wall. In contrast, only 5–12 ganglia contained 3–12 acetylcholinesterase-positive nerve cell bodies at the same foetal age. No catecholamine-containing nerve cell bodies were seen at any foetal age or in the adult and catecholamine-containing nerve fibres were not detected until 28 days of gestation. In adult bladder, there was a reduction of 25–30 % in the number of quinacrine-positive cell bodies within the ganglia when compared with 1-day-old bladders, although there was an increase of about 50 % in nerve fibres.
The postnatal development of purinoceptors in rat urinary bladder has also been examined. Neurogenic contractions of bladders from newborn rats were atropine-sensitive in the whole range of frequencies studied. During the first 2 weeks, the atropine-resistant component of these contractions increased progressively to reach adult-like conditions, i.e., atropine-resistant contractions consisted of over 90 % of contractions at 0.1 Hz and about 60 % at 1–20 Hz [462]. Responses to adenosine (inhibitory) and ATP (excitatory) mediated by P1 and P2X receptors, respectively, were present as early as postnatal day 2, the earliest day studied [520]. Adenosine was more potent in the neonate than in the adult, while the potency of ATP initially increased with age, but then declined, being highest between postnatal days 10 and 25. In vivo evidence for the functional roles of cholinergic and purinergic components of parasympathetic cotransmission for micturition contractions in normal unanaesthetised rats has been presented [326].
Western blot studies demonstrated an age-dependent decrease in P2X2 receptor expression in the postnatal bladder, whereas P2X3 receptor expression peaked at P14–P21 [638]. P2X2 immunoreactivity was present in urothelial cells, suburothelial sensory plexuses, smooth muscle and serosa in bladder neck and trigone at birth. With increasing postnatal age, the intensity of P2X2 immunoreactivity decreased in urothelial cells but increased in the suburothelial plexuses, while P2X3 immunoreactivity increased in urothelial cells and suburothelial plexuses with postnatal age. P2X3 receptor expression in dorsal horn of the lumbospinal spinal cord also increases from P14 to P21. Importantly, the authors suggest that these changes in P2X receptor expression may play a role in the postnatal maturation of voiding reflexes. It is known that during postnatal maturation a spinobulbospinal reflex and voluntary voiding replaces premature voiding reflexes.
The main pathway in nerve activation of the urinary bladder of newborn mice is cholinergic with a low contribution of the purinergic component, while adult bladder is equally dependent on cholinergic and purinergic activation [224]. However, there were no differences in responsiveness of new-born and adult bladders to ATP or α,β-meATP, suggesting that the differences in purinergic control between new-born and adult bladders was due to the properties of ATP transmitter release rather than to a change in receptor function.
Using various markers for sensory and motor nerves, it was concluded that both nerve types were present at birth and that sensory (calcitonin gene-related peptide [CGRP]-positive and SP-positive) nerve fibres approached adult levels at the end of the second week, shortly before the micturition reflex was fully developed [594]. In a review of postnatal development in several rat visceral smooth muscle preparations, it was concluded that in the bladder, in contrast to vas deferens, purinergic mechanisms were more important in the neonate than in the adult [304].
In foetal human bladder, expression of P2X1 receptor transcripts was much lower than in adult bladder; P2X4 and P2X7 receptors were also present in the foetus [536]. With increasing gestation, the P2 receptor expression shifted from the dome to the body of the bladder. Obstruction of the foetal male sheep bladder leads to enlarged, hypocontractile and compliant bladder; however, there was no clear evidence for changes in purinergic (or in cholinergic or nitrergic) neurotransmitter effects [673].
The rate and pattern of breakdown of ATP and adenosine by ectoenzymes in the rat urinary bladder was shown to be identical in neonates and adults, indicating that the marked differences in potency to ATP and adenosine during development is likely to be due to changes in receptor number and/or agonist potency [522]. The distribution of P2X receptors on smooth muscle cells during postnatal development has been studied [220]. Small clusters of P2X receptors (about 0.4 μm in diameter) were present at day P1, although few varicose nerve fibres were present at this time. At P4, many varicose fibres were present and small clusters of P2X receptors; some appeared to be in association with varicosities. By P21, many of the P2X receptor clusters were found adjacent to varicosities of parasympathetic nerve fibres, but others were not. Newborn rat detrusor smooth muscle showed markedly increased purinoceptor-mediated contractions, which reached adult levels 1 month after birth [683].
Ageing
There are few reports describing changes in purinergic signalling in the ageing bladder, although a comparison of contractions in detrusor muscle strips from unobstructed bladders of young and aged rats showed that, with age, there is an increased sensitivity to ATP as well as NA, but with no change in response to ACh and KCl [236]. The secondary role of PGs in response to purinergic transmission appears to be increased in old age [353]. A reduction in acetylcholinesterase-positive nerve fibres in the human bladder with increasing age has been reported [266] and decreased fluorescence intensity for catecholamines in neurons in the hypogastric ganglion which supplies sympathetic fibres to the bladder has also been shown [550]. Ageing impairs neurogenic contractions mediated by ATP, but less so ACh, in guinea-pig urinary bladder; melatonin has been used to improve these age-induced changes [269].
It has been reported that P2X3 receptor expression is increased after ovariectomy and the authors speculated that this may explain why there is an increase in bladder dysfunction in ageing women [676].
The contractile response of the rat bladder to ATP released as a cotransmitter from parasympathetic nerves increases with age [353]. The contractile responses of the aged rat bladder to ATP are significantly greater than those of the young bladder, although there is no change in the responses to ACh or KCl [587]. The atropine-resistant (purinergic) component of nerve-mediated contractions of the human bladder was also increased with age, largely due to increased release of ATP [739]. The sensitivity of the bladder to α,β-meATP increased with age [729]. However, the mRNA detected for P2X1 and P2X3 receptors did not change with age, although there could be changes in receptor protein. For the ageing rat bladder, increased expression of P2Y4 receptors was reported [639].
Purinergic transmission increases with age in the human bladder, while cholinergic transmission decreases. These effects appear to be due to increased release of ATP and decrease in ACh release and the authors suggest that purinergic receptor antagonists may provide a useful complement to muscarinic receptor antagonists in the treatment of older patients with overactive bladder (OAB) [739]. Ageing impaired the contractile response of mouse detrusor muscle strips, but responses to ATP were enhanced [270]. There is down-regulation of P2X1 mRNA expression with age in the detrusor muscle of male patients with and without bladder outlet obstruction [154]. They suggest that P2X receptor mRNA downregulation may occur as the result of increased neural ATP release in the ageing bladder.
Plasticity of purinergic signalling in bladder
Changes occurring during pregnancy or hormone therapies
Incontinence is a common problem in adult women [684]. The first symptoms of urinary incontinence can arise after the first pregnancy and the risk of incontinence increases with multiple deliveries [584]. However, the sensitivity of the rat detrusor muscle to ATP was not modified by multiple pregnancies, while there was increased sensitivity to adrenergic and cholinergic stimulation [273]. Other studies reported that the responses to adrenergic and cholinergic stimulation were reduced [422, 679], and the responses to ATP increased during pregnancy in both rat and rabbit bladders [679]. These latter authors concluded that suppressed bladder contractility during pregnancy, due to a reduction in cholinergic and less importantly α-adrenergic function, is associated with decreased muscarinic receptor density, while the affinity of purinergic receptors for ATP is increased. The sensitivity of the detrusor muscle to α,β-meATP was not modified by multiple pregnancies, even though smooth muscle hypertrophy occurred [273].
An old concept is that incontinence during pregnancy is related to hormonal factors [334, 634]. Oestrogen has been used for the treatment of urinary stress incontinence in women [91, 591]. Oestrogen is known to have a profound influence on the function of smooth muscle [47] and receptors for oestrogen have been identified in both rabbit myometrium [46] and human female lower urinary tract [334]. When ovariectomized rabbits were injected i.v. with [3H]oestradiol, high affinity binding sites were clearly demonstrated in the female urethra and urinary bladder [334]. The amplitude of NANC transmission in detrusor strips from mature female rats was increased in oestrogen-treated, but not ovariectomized animals [223].
Sex hormones influence detrusor responses to purinergic stimuli and alteration of sex hormone levels by surgery or medication modulate bladder function, which are different in males and females [671]. Chronic treatment with oestrogen induced a marked increase in the responses to purinergic (as well as muscarinic and α-adrenergic) agonists in the rabbit bladder body and mid-section, but not the bladder base [421]. Pregnancy substantially increases the purinergic components of the response of the rabbit bladder to field stimulation, while the response of bladder to bethanechol was significantly reduced and was associated with a 50 % decrease in muscarinic receptor desensitisation [424]. Oestradiol and the oestrogen receptor antagonist, tamoxifen, inhibit contractions of rabbit detrusor strips produced by α,β-meATP and bethanechol [574]. After ovariectomy, there was an increase in P2X3 receptor mRNA expression in the bladder [129]. Progesterone administration mimics some, but not all, of the effects of pregnancy; for example, no significant alteration in the response to ATP was observed [679].
Degeneration of adrenergic nerves in the rat urinary bladder during pregnancy has been described [570]. Since ATP is a cotransmitter in sympathetic nerves, it is likely that less ATP as well as NA is available in pregnant compared to non-pregnant bladders.
Changes due to selective denervation
When the rat urinary bladder was deprived of half of its innervation by removing the pelvic ganglion on one side, the motor responses of the bladder to stimulation of the remaining pelvic nerve were larger than those of the control bladder at 1 week, 1 month and especially 2 months postoperatively [225]. Further experiments with atropine and eserine led to the conclusion that the increased response 1 week postoperatively were mainly due to sensitisation of the muscarinic receptors, while those observed at later stages were due to collateral sprouting from the cholinergic nerve fibres in the intact pelvic nerve. Atropine-resistant responses were not examined. In a later study, development of supersensitivity to methacholine in rat detrusor following either parasympathetic denervation or decentralisation was reported [227]. When the sacral parasympathetic preganglionic pathways were surgically interrupted on one side of the cat urinary bladder, it was claimed that cholinergic sympathetic pathways in the hypogastric nerve make sympathetic connections with decentralised cholinergic ganglion cells in the bladder [203].
Capsaicin treatment of newborn rats leads to selective degeneration of sensory nerve fibres (see [302]). In a study of rat bladder in 3-month-old rats treated at birth with capsaicin, contractions evoked by electrical field stimulation were significantly larger than those of control (vehicle-treated) animals, an effect which preferentially involves the cholinergic component of the response, although there was some increase, too, in the purinergic component [765]. However, since contractions in response to exogenous carbachol or ATP were not significantly different, this suggested that the changes involve prejunctional mechanisms, probably a trophic increase in parasympathetic innervation. Capsaicin treatment, causing selective sensory denervation of the rat ureter, leads to increased sympathetic innervation [593] presumably leading to increase in release of both NA and ATP.
After bilateral sympathectomy by cutting the hypogastric nerves distal to the hypogastric ganglia, the adrenergic nerve supply to the bladder did not differ from normal bladder either at 10 days or 6–9 weeks after denervation; in contrast, 10 days after total unilateral postganglionic denervation by removal of the left pelvic ganglion, few adrenergic nerve fibres were seen in the left half of the bladder [17]. However, 6–9 weeks after pelvic ganglion removal, the adrenergic innervation had reappeared, although the origin of the regenerating fibres was not resolved. Studies on the vascular system show that P1 receptor agonists prevent the trophic changes caused by sympathetic denervation, which was taken to be consistent with an involvement of purines in the trophic effects of sympathetic innervation [13]. In spontaneously hypertensive rats, where there is increased sympathetic nerve activity, there is hyperactive bladder voiding that appears to be associated with higher secretion of NGF by bladder smooth muscle and hyperinnervation [156, 633].
Over-distension of the bladder is caused by urinary retention, but it has also been used as a method for treating unstable bladder or IC [218, 348]. However, micturition problems are often encountered after long-term over-distension [658] possibly due to damage of sensory nerve fibres. For example, distension of the rat urinary bladder for 3 h led to depletion of catecholamines which was complete after 2 days, although partially recovered after 5–7 days [659]. The urinary bladder of the rat, deprived of its motor innervation, increases several-fold in weight in response to distension [228]. This increase in weight is due to both hyperplasia and hypertrophy of the smooth muscle [226]. Since it is now known that distension of the bladder leads to substantial release of ATP from urothelial cells and ATP is known to have trophic effects [2], it is possible that purines participate in the trophic changes that occur in the bladder.
Damage to the spinal cord rostral to the LS level can induce marked changes in the neural control of the lower urinary tract; following spinal cord injury that interrupts the normal supraspinal pathway regulating micturition, the urinary bladder is initially areflexic, but over the course of several weeks becomes hyperreflexic and hypertrophic [204, 387]. Little is known about the mechanisms underlying these changes [204], although it has been shown that chronic spinal injury enhances the electrical excitability of bladder afferent neurones by increasing the expression of low-threshold tetrodotoxin-sensitive Na+ channels [742].
Bladder grafts
The gastrointestinal tract has been the chief source of material for bladder augmentation and substitution despite complications such as malignancy, electrolyte abnormalities, infection, obstruction, the inherent need for catheterisation, mucus production and perforation (see [490]). A small intestine submucosal preparation has been developed and used as a bladder patch in rats that produced both smooth muscle and urothelial cell regeneration [385, 386]. A further study indicated that small intestine submucosal-regenerated bladder exhibits contractile activity, expresses muscarinic, purinergic and β-adrenergic receptors and exhibits functional cholinergic and purinergic transmission [697].
Another approach has been to use autologous cultured urothelium for bladder reconstruction [40, 324]. Collagen-based and biodegradable materials have also been shown to have regenerative and functional capacities and a bladder acellular matrix graft has been claimed to be successful for augmentation cytoplasty in the rat model leading to structural and functional regeneration of detrusor smooth muscle [568] including contractile activity to electrical field stimulation showing responses to muscarinic, purinergic and adrenergic agonists [557].
Hibernation
Purinergic and cholinergic components of parasympathetic neurotransmission were investigated in hibernating hamsters (Fig. 5) [560]. Perhaps surprisingly, 4 weeks of hibernation significantly increased both cholinergic and purinergic neurogenic responses of the hamster urinary bladder. This appears to be due to an increase in postjunctional responses to ACh, while there was a decrease in the postjunctional response to ATP.
Non-cumulative concentration–response curves to α,β-methylene ATP (α,β-meATP) in longitudinal smooth muscle strips of urinary bladder from age-matched control hamsters, cold-exposed hamsters and hibernating hamsters. In cold-exposed and hibernating hamsters α,β-meATP elicited decreased contractions (ANOVA, P < 0.05; n = 6). (Reproduced from [560], with permission from Wiley)
Purinergic signalling in the human bladder in health and disease
Although the purinergic component of parasympathetic neuromuscular transmission in the urinary bladder is between 40 % and 75 % in laboratory animals, in normal human bladder, atropine will block over 95 % of parasympathetic nerve-mediated contraction, despite the fact that P2X receptors are present [106]. However, there are a number of examples where the purinergic component of cotransmission is increased to up to 40 % in pathological conditions such as IC [540], outflow obstruction [537, 623], idiopathic detrusor instability [537] and some types of neurogenic bladder [27].
Recent reviews of management of detrusor dysfunction highlight the growing potential of therapeutic strategies related to purinergic signalling (e.g., [27, 64, 239–241, 243, 247, 496, 572, 582, 744]).
Healthy bladder
The presence of an atropine-resistant nerve component in the human bladder has been controversial, although most authors did find a small component, usually less than 5 % in healthy bladder [31, 50, 166, 262, 296, 371, 446, 462, 518, 583,611, 620, 631, 652]. One early report suggested that the NANC component in the human female bladder was greater than in the male bladder, amounting to about 50 % of the nerve-mediated contractile response of the bladder [163]. In a paper concerned with anticholinergic drugs, it was claimed that terodiline and propiverine significantly inhibited the atropine-resistant contractions in the human bladder [707].
Atropine-resistant responses of the human bladder were significantly reduced by ATP (possibly mediated by postjunctional desensitisation and/or prejunctional inhibition) and indomethacin and were abolished by nifedipine [323]. One paper claimed that the atropine-resistant component of excitatory transmission in the human bladder was not mediated by neural release of ATP in spite of the presence of P2 receptors in the effector cells [652].
A NANC nerve-mediated relaxation following the initial excitation was identified in human detrusor muscle [378]. Transmural stimulation of muscle strips from the human trigone revealed a NANC response which represented 40 % of maximal contractions at 5 Hz; NANC relaxation responses were also identified in the trigone [631] and in detrusor where they might be due to NO [339]. Responses of human bladder strips to NANC nerve stimulation and ATP or P1P6-diadenosine hexaphosphate were blocked following desensitisation of P2X1 receptors with α,β-meATP [313] (Fig. 6).
Responses to ATP, α,β-methylene ATP (MeATP) and Pl,P6-diadenosine 5′-hexaphosphate (A6PA) in isolated human urinary bladder detrusor muscle. a Concentration–response relationships. The response curve relates contractions due to the agonists to the standard contraction to KC1 (150 mM). Points show mean ± SEM unless occluded by symbol. Curves are fitted following probit transformation and horizontal averaging. b Electrical field stimulation of the intramural nerves (NS, filled circle) evoked contractions. MeATP (0.3 μM) caused a small contraction which faded and blocked neurogenic contractions. Following washout of MeATP (W), the neurogenic responses returned. Record obtained in the presence of atropine (0.3 μM). Scale bar represents 50 mg. (Reproduced from [313], with permission from Elsevier)
There is clear evidence for the presence of P2X purinoceptors in the human bladder from pharmacological studies where ATP produces contractions [313, 323, 333]. ATP, α,β-meATP and P1P6-diadenosine hexaphosphate caused concentration-dependent contractions of human detrusor muscle strips [313]. ATP elicits large inward currents [333] and increases in intracellular Ca2+ [50, 728] in dispersed human bladder smooth muscle cells. In a study of P2 receptor subtypes in human bladder strips the agonist rank order of potency was: α,β-meATP = ADPβS > 2-MeSATP > ATP >> UTP. In addition, it was reported that responses to α,β-meATP and ADPβS were additive and that the P2 antagonist p-chloromercuribenzene sulphonic acid [724] antagonised ADPβS-induced contractions, but was inactive against α,β-meATP, while Reactive blue 2 had no effect against ADPβS contractions [541]. The authors concluded that the human detrusor muscle contains two contractile purinoceptor subtypes: one is activated by α,β-meATP and is probably a P2X receptor; the other receptor is activated by ADPβS and appears to be different from those which are included in the current classification system. In a later paper from this group [542], evidence was presented for prejunctional P2 receptors on parasympathetic nerve terminals as well as two postjunctional P2 receptor subtypes, one of which was insensitive to suramin. ATP-induced contractions were reduced about 30 % by indomethacin, indicating involvement of PGs, by 48 % after nifedipine and were abolished in Ca2+-free medium [323].
Supporting evidence for P2X receptors in human bladder comes from radioactive ligand binding and autoradiography [74,487], and from immunohistochemistry [537]. Additionally, a cDNA encoding an ion channel receptor (hP2X1), gated to extracellular ATP, was isolated from human urinary bladder [695]. By fluorescence in situ hybridisation, the hP2X receptor gene was mapped to the short arm of human chromosome 17. ATP stimulated NGF production in human bladder smooth muscle cells, which suggests that the human bladder may have a mechanism to maintain and regulate the extent of bladder innervation [661].
The main source of ATP release from the human (and pig) bladder is urothelium to act on subepithelial sensory nerve terminals [395]. The vesicular nucleotide transporter plays a crucial role in stretch-evoked release of ATP from human bladder urothelium [511]. Bradykinin increases NGF mRNA expression in the human urothelial cell line (UROtsa) and stretch-induced ATP release [532]. It was claimed that adenosine formation from extracellular ATP was negligible in human urinary tract urothelial cells due to low CD39 expression, but the cells express CD73, which converts extracellular AMP to adenosine [492]. Expression of P2X3 receptors has been described on suburothelial myofibroblasts of the normal human urinary bladder [438]. ATP enhances spontaneous calcium activity in cultured suburothelial myofibroblasts of the human bladder, supporting the notion that suburothelial myofibroblasts are able to register bladder fullness [145]. Different signalling pathways for A2A and A2B adenosine receptors expressed by human uroepithelial cells were identified [598]. Mechanical stretch (which presumably leads to release of ATP) promotes contraction and proliferation of human bladder smooth muscle cells via P2X and perhaps A1 or P2Y receptors, respectively [715].
Overactive bladder syndrome
OAB syndrome is characterised by urgency, with or without urge incontinence (sometimes referred to as OAB wet and OAB dry, respectively), frequency and nocturia [3]. In OAB dry patients the urgency is accompanied by discomfort or pain during filling, and this is defined as BPS. Some patients with BPS are later diagnosed with IC.
The occurrence of OAB increases in old age. Bladders of 24-month-old mice showed a significantly higher afferent response to distension compared to 3-month-old mice and ATP detected in intraluminal samples was higher in the old mice [187]. However, the underlying mechanisms mediating these findings and the increased occurrence of OAB were not resolved. Valuable reviews concerning the roles of purinergic signalling in OAB are available [36, 41, 109, 110, 239, 398,408, 484, 538].
In recent papers it was reported that women with OAB have high urinary levels of ATP compared to controls and the nucleotide levels increase with water intake and it was suggested therefore that higher urinary ATP may be a useful prognostic marker for detrusor overactivity [142, 615]. It was suggested that the increased concentrations of ATP in the urine is probably due to enhanced ATP release from proliferating urothelium and that decreased ATP metabolism may also contribute to the high urine levels of ATP [615]. The ATP concentration in urine was inversely related to the volume at the first desire to void in women patients with OAB [142]. Both TRPV1 and P2X3 receptors are present in the human bladder and it was suggested that they may become upregulated and contribute to distinct pathophysiological states of OAB [735].
People with metabolic syndrome, a condition characterised by an increased risk of developing cardiovascular disease and diabetes, have a higher prevalence of OAB. Using a rat model of metabolic syndrome, fructose-fed rats, alterations in peripheral purinergic and muscarinic signalling were described [155]. It was claimed that there was an increase in expression of P2X3 receptors in subepithelial sensory nerves leading to increased bladder activity [413]. OAB is prevalent among patients with Parkinson’s disease and suppression of this overactivity by A2A receptor antagonists has been reported, which are probably acting in the CNS to regulate the micturition reflex [375].
UDP via P2Y6 receptors regulates abnormal bladder smooth muscle activity in OAB and in doing so enhances P2X1-mediated contractions [751]. Iberiotoxin, a bradykinin receptor antagonist, enhanced purinergic contractions, suggesting that bradykinin receptors in bladder play a significant role in OAB [675].
Detrusor overactivity
Detrusor overactivity (previously termed unstable bladder/detrusor instability and detrusor hyperreflexia) is characterized by involuntary detrusor contractions during the filling phase that are either spontaneous or provoked. Detrusor overactivity can be either neurogenic or idiopathic in origin [3].
Neurogenic detrusor overactivity
Neurogenic detrusor overactivity is defined as a known neurologic abnormality impairing signalling between the bladder and the CNS. This can occur following a stroke, or spinal cord or pelvic injury and in conditions such as multiple sclerosis (MS) and Parkinson’s disease.
Studies have been conducted on isolated bladder strips from patients with neurogenic bladder who underwent ileocystoplasty in order to resolve intractable incontinence and/or vesicoureteric reflux due to low compliance or severe detrusor uninhibited contractions [589]. Atropine-resistant responses to field stimulation of neurogenic bladder strips were about 30 % compared with 4 % from control bladder strips. In a later paper, this group showed that neurogenic bladders are hyper-responsive to ATP [588]. In another study of muscle taken from neurogenic bladders, a NANC component of 40 % was identified, which was regarded as purinergic since it was blocked by suramin [710].
Both resting and evoked ATP release from urothelial cells was significantly higher after chronic spinal cord injury [644]. In paraplegic patients with suprasacral lesions, the management of urinary incontinence resulting from hyperreflexic detrusor contraction is a frequent problem. A study of changes in cholinergic and purinergic transmission was carried out in detrusor muscle strips taken from chronic spinal rabbits (spinal cord transected at thoracic level T9–T10) with detrusor hyperreflexia and detrusor sphincter dyssynergia [736]. The results showed that the relative amplitudes of the cholinergic and purinergic contractions shifted from a control ratio of 40:60 to 75:25 in the pathologic detrusor, indicating a shift to cholinergic parasympathetic dominance in neurogenic bladders affected by detrusor hyperreflexia and sphincter dyssynergia after spinal cord injury. Increased P2X2 expression in both detrusor muscle and urothelium from patients with suprasacral spinal cord injury appear to be comparable to their expression in detrusor tissue from patients with idiopathic OAB [544]. Increased levels of ATP in the bladder of spinal cord-injured rats are associated with neurogenic bladder overactivity and the activation of P2X3 and P2X2/3 receptors on afferent nerve fibres [507]. The carbachol-induced inhibition of parasympathetic NANC purinergic contractions mediated by P2X1 receptors occurs more in rat bladders following chronic spinal cord injury than in neurally intact animals [403]. Spinal cord injury leads to an increase in spontaneous contractile activity and propagating Ca2+ waves in the bladder and these activities are augmented by the P2Y receptor agonists ADP, UTP and UDP originating in the urothelium and suburothelium [250].
The induced synthesis of PGs may become important in pathological conditions. For example, in patients undergoing retropubic prostatectomy, the detrusor has a larger non-cholinergic excitatory component than in patients undergoing cystourethectomy [323]. In the former group, indomethacin causes a significant reduction of the response to non-cholinergic nerve stimulation, whereas in the latter group, indomethacin has no such effect. In patients with chronic neurogenic vesical dysfunction, the sensitivity of the bladder to intravenous infusion of an analogue of PGF2α is dramatically greater than in control patients [692]. The significance of this is unknown, but in view of the relationship of ATP with PG synthesis, it may be related to a degeneration of parasympathetic nerves resulting in supersensitivity to effectors. That is to say, a loss of purinergic transmission might have led to an increase in sensitivity of its effector mechanisms, one of which is PG activity [311].
Reflex sympathetic dystrophy is a disabling syndrome characterised by severe pain with autonomic disturbances, including urological problems [135, 257, 601]. Since hyperactivity of sympathetic nerves is usually implicated in reflex sympathetic dystrophy, more ATP would be released as a cotransmitter to target both P2X1 receptors in smooth muscle mediating bladder contractions and P2X3 receptors on the terminals of sensory nerve fibres mediating bladder reflexes and nociception.
Animal models have been used to investigate bladder overactivity. For example, both resting and evoked ATP release from urothelial cells were significantly higher in the bladder of rats after chronic spinal cord injury and may contribute to the development of bladder hyperactivity [368]. In a mouse model of bladder overactivity, bradykinin was shown to facilitate the release of ATP from nerve terminals via prejunctional receptors [234]. In the mouse spinal cord transection model of detrusor overactivity, there was an increase in the amplitude of spontaneous contractions and it was suggested that enhanced stretch-induced urothelial ATP release is implicated in the increased spontaneous contractions as well as the enhanced afferent firing seen in bladders of mice with spinal cord transection [475]. Antimuscarinic agonists suppressed ATP release from the urothelium and improved detrusor overactivity in rats with cerebral infarction [737].
In the absence of P2X3 receptors in mouse knockouts, the bladder is hyperactive [158,704]. The more recently developed P2X3 and P2X2/3 antagonist, AF-219, which is orally bioavailable and metabolically stable, is being explored as a therapeutic agent for urinary tract dysfunction [239]. The isolated porcine detrusor has been claimed to be a reliable model for the development of novel, selective P2X3 receptor antagonists for the treatment of detrusor hyperactivity [182].
The use of ketamine as a recreational drug, particularly in adolescents, is widespread, but has side effects that include increased voiding frequency, urgency, dysuria, nocturia and decreased capacity. Mice treated with ketamine for 8 weeks showed similar side effects and enhanced P2X1 receptor expression and non-cholinergic nerve-mediated contractions were demonstrated [483]. It was suggested that dysregulation of purinergic neurotransmission may underlie detrusor overactivity in ketamine-induced bladder dysfunction.
Idiopathic detrusor overactivity
In cystometrically verified unstable human bladder, a varying degree of atropine resistance was described, with some preparations showing a 50 % resistance to atropine [620]. Atropine-resistant nerve-mediated contractions have been demonstrated in hypertrophied bladders, secondary to benign prostatic hyperplasia and it was suggested that the NANC component might be related to the hyperactivity observed in these bladders [583, 620]. Increased atropine-resistant nerve-mediated responses have also been shown in the bladders of myelodysplastic children [262]. This finding was supported in a later study where, in contrast to control bladders, a NANC excitatory response amounting to about 25 % of the total nerve-mediated contraction was described in strips from bladders obstructed by benign prostatic hyperplasia [623].
In a study of human detrusor muscle, it was reported that the purinergic atropine-resistant contraction was prominent in obstructed or unstable bladders but not those with neurogenic instability. This change was not caused by a differential sensitivity of the muscle to ATP or cholinergic agonists [50]. In a follow-up paper by the same group [725], it was confirmed that the generation of purinergic contractions in detrusor strips from unstable bladders was not due to altered sensitivity of detrusor muscle to ATP. P2X1 receptor subtype expression was markedly increased in human unstable bladders [537]. A further possible explanation for the increased potency of ATP in generating contractions in detrusor from unstable bladders may be reduced extracellular ATP hydrolysis [281]. Reduction of P2X3 and P2X5 receptors in human detrusor from adults with urge incontinence has been claimed [495]. The possibility that the increase in purinergic transmission is due to increased neural release of ATP, reduction in ecto-ATPase activity or to changes in gap junctions between muscle cells has been raised [50, 249]. On the other hand, an earlier study [323] showed that preparations obtained from hypertrophic human bladders were more sensitive to ATP than macroscopically normal preparations. ATP released from the urothelium may mediate initial afferent sensation in patients with detrusor overactivity characterised by urgency [143].
It has been claimed that loss of smooth muscle caveolae-mediated regulation of purinergic signalling and augmented spontaneous activity in spontaneously hypertensive rats leads to detrusor overactivity [171].
In patients with idiopathic detrusor instability, there is abnormal purinergic transmission to the bladder; this may account for some of the symptoms of OAB [27, 537]. An increase in density of subepithelial sensory nerves has been described in the bladder wall of women with idiopathic detrusor instability; the authors speculated that this may serve to increase the appreciation of bladder fitting, giving rise to the frequency and urgency of micturition which are characteristic of patients with detrusor instability [494]. The greater potencies of ATP for generating contraction in detrusor in patients with unstable bladders may be due to reduced extracellular hydrolysis by ectonucleotidases, allowing greater access of ATP to detrusor smooth muscle P2X1 receptors [281]. Connexin 45 is expressed at low levels in the smooth muscle of the bladder detrusor, but is significantly up-regulated in women with urge symptoms and its relationship with purinergic signalling discussed [519]. ATP is released during cystometry in women with detrusor overactivity and BPS and may contribute to urgency [141]. Release of ATP from the urothelium of human bladders with detrusor overactivity has been reported for both neurogenic and idiopathic conditions [396]. Rat bladder overactivity induced by intravesical instillation of ATP with protamine sulphate pretreatment was reduced by PPADS as well as oxybutynin that is not acting as an antimuscarinic [493]. Anticholinergics, such as oxybutynin, are widely used for the treatment of patients with OAB, about 90 % of whom suffer from idiopathic OAB. A recent study has shown that chronic administration of oxybutynin induces a shift from muscarinic to purinergic transmission in the bladder wall of rats and it was suggested that this may partially explain the high discontinuation rate of anticholinergics used for the treatment of OAB [691]. Increased expression of P2X3 receptors on suburothelial sensory nerve fibres has been observed in patients with idiopathic detrusor overactivity [434].
Bladder pain syndrome/interstitial cystitis
BPS is pain of unknown aetiology related to bladder filling together with other symptoms, such as increased day-time and night-time frequency, in the absence of proven urinary tract infection or other pathogens [300, 383]. While this definition also includes IC, the term IC is usually only applied to patients with typical cystoscopic and histologic features [192]. A recent review includes discussion of the possible roles of ATP and adenosine in BPS/IC [699].
In detrusor strips taken from patients with IC, the atropine-resistant contractile component was about 43 % of the total responses, while this component was not observed in controls [540]. Parasympathetic nerve-mediated contractions of the rat bladder, evoked by the release of ATP and ACh, were substantially reduced in cystitis induced by cyclophosphamide [701]. The NANC (purinergic) component in the neurogenic bladder was abolished following desensitisation with α,β-meATP and the detrusor muscle showed increased sensitivity to the agonist actions of α,β-meATP, in contrast to decreased sensitivity to ACh and histamine. The A1 receptor is present in rat urinary bladder and is decreased in cyclophosphamide-induced cystitis and mediates relaxation, perhaps replaced by the A2B receptor, while A3 receptors mediate contraction [700]. Stimulation of A1 receptors at the luminal surface of the urothelium stimulates voiding in animals with cyclophosphamide-induced cystitis [567].
Stretch-activated ATP release from bladder epithelial cells from patients with IC is significantly greater than from healthy cells [647], as well as in the cat model of IC [63] and in cyclophosphamide-induced cystitis in rats and mice [626]. The P2X3 receptor subunit was upregulated during stretch of cultured urothelial cells from patients with IC [645]; P2X2 and P2X3 receptor expression has been demonstrated on human bladder urothelial cells (as well as on afferent nerve terminals); the expression was greater in cells from IC bladder [63, 662]. ATP-stimulated ATP release is augmented in IC bladder urothelial cells compared to healthy urothelial cells [648]. They showed further that ATP-stimulated release of ATP from healthy urothelial cells can be induced by treatment with epidermal growth factor and that P2X3 receptors are increased. Upregulation of afferent nerve fibres in IC has also been claimed [516].
Detrusor overactivity is induced by intravesical application of ATP. It was suggested that enhanced penetration of endogenous ATP due to urothelial damage may contribute to urinary frequency and bladder pain in hypersensitive bladder in BPS/IC [524]. Urothelial cells from patients with IC released significantly more ATP in response to stretch than did control cells [644]. Further, the authors showed that the stretch-activated ATP release was blocked by adding dimethyl sulphoxide or heparin, both intravesical agents commonly used to treat the symptoms of IC.
The P2X3 receptor in rodents is largely expressed in the so-called IB4-labelling small nociceptive capsaicin-sensitive nerves in the DRG, so it is interesting that IB4-conjugated saporin, a cytotoxin that destroys neurons binding IB4, when administered intrathecally at the level of L6–S1 spinal cord, reduced bladder overactivity induced by bladder irritation by ATP infusion [525]. The authors suggest that targeting IB4-binding, non-peptidergic afferent pathways sensitive to capsaicin and ATP may be an effective treatment of overactivity and/or pain responses of the bladder. Bladder urothelial cells from patients with IC have augmented extracellular ATP signalling that could be blocked by suramin and heparin-binding epidermal growth factor-like growth factor [646].
Cats suffer a naturally occurring chronic idiopathic cystitis, termed feline IC (FIC), with features similar to human IC [409]. Intracellular Ca2+ measurements in cultured urothelial cells revealed that purinergic responses of the urothelium are changed in FIC [96]. Urothelial cells from normal cats showed increased intracellular Ca2+ levels in response to 2-MeSATP but not to α,β-meATP, suggesting the presence of P2Y but not P2X receptors in normal tissue. However, urothelial cells from FIC cats responded to 2-MeSATP and to α,β-meATP, indicating the increased expression of P2X receptors in the urothelium of animals with the disorder. NO release was also altered in FIC [232]. Capsaicin-induced release of NO was reduced in the mucosal strips from FIC cats compared to controls, whereas basal NO release mediated by inducible NOS was increased in FIC cats. Increased expression of c-jun and NK2 receptors was also noted in bladder afferent neurones in FIC cats. These results indicate chemical signalling in the urothelium and in bladder sensory nerves is altered in chronic cystitis in cats. FIC results in mechanical hypersensitivity and increased ATP release from bladder urothelium [58]. Also, there is a marked reduction in P2X1 and a loss of P2Y2 receptor staining throughout the urothelium [63]. In cyclophosphamide-induced cystitis in rats, there are substantial changes in both sympathetic and parasympathetic efferent nerves, which affect the afferent nervous input from the bladder; changes in parasympathetic innervation occur prejunctionally, while changes in sympathetic innervation postjunctionally [263]. It has been reported that hyaluronic acid reduced bladder hyperactivity by inhibiting H2O2-enhanced purinergic and muscarinic signalling [734].
A model for BPS/IC has been developed in rats by administering cyclophosphamide, an anticancer drug which is metabolised in the body to acrolein, a chemical irritant that is excreted in the urine. Acrolein enhances ATP release and reactive oxygen species formation in cultured human urothelial cells [149]. Rats treated with cyclophosphamide develop characteristic behavioural signs associated with bladder pain in parallel with the development of bladder lesions and increased expression of immediate early gene-encoded proteins c-fos and Krox-24 in the spinal cord [407, 703]. In bladder afferent neurones, a marked increase in the excitability [743] and in the expression of neuronal NOS [703] has also been detected after cyclophosphamide treatment, indicating a change in the electrical and chemical properties of bladder afferents following chronic inflammation. It would be interesting to see if any changes in motor and/or sensory purinergic signalling occur in this model. Another model that shows many of the characteristics of BPS/IC has been proposed, where hypersensitivity inflammation of the bladder in vivo is induced by local application of ovalbumin in ovalbumin-sensitive female rats [8]. Based on cyclophosphamide-induced inflammatory cystitis, it was suggested that A1 receptor blockade during the initial phase of BPS/IC may be a future treatment for this disease [38]. Cyclophosphamide-induced bladder inflammation sensitises and enhances P2X receptor function in rat bladder sensory neurons [190]. LS and TL neurons from cyclophosphamide-treated rats showed a selective increase in the functional expression of both P2X2/3 and P2X3 receptors. TRPV1 is involved in generating bladder noxious sensory input and the high frequency reflex contractions that occur during cystitis [136]. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release from urothelial cells [491]. In rats with cyclophosphamide-cystitis, there is a down-regulation of both muscarinic and purinergic receptors in the bladder, perhaps induced by the enhanced activity of both cholinergic and purinergic nerve activity occurring subsequent to cyclophosphamide treatment [352]. Targeting deletion of Dicer, an enzyme essential for microRNA processing perhaps including those targets of P2X receptor mRNAs, exacerbated cyclophosphamide-induced OAB [757]. The selective inhibitory effect of NO in cyclophosphamide irritated bladders may be due to a suppression of purinergic excitatory mechanisms in bladder sensory pathways following ATP release from urothelial cells and activation of P2X3 receptors on afferent nerve endings [748].
Purinergic agonists acting on P2X receptors on the urothelium or directly on suburothelial afferent axon terminals can increase the excitability of afferent nerves. Cystitis induced by cyclophosphamide pretreatment can mimic the sensitizing effect of purinergic agonists, consistent with the evidence that ATP is involved in nociceptive mechanisms in the urinary bladder [747]. Subsensitivity of P2X3 and P2X2/3 receptors, but not vanilloid receptors, has been shown in L6–S1 DRG in the rat model of cyclophosphamide cystitis [79]. Release of ATP from urothelial cells with hypoosmotic mechanical stimulation was increased by over 600 % in inflamed bladder from cyclophosphamide-treated animals; BTX inhibited this release [626]. In rats with hydrochloric acid-induced cystitis, there was a loss of both muscarinic and purinergic receptors, although the in vivo release of ATP from mucosal cells was significantly enhanced, perhaps acting on P2X3 receptors on afferent fibres to contribute to urinary frequency and non-voiding contractions [738, 740], as well as in cyclophosphamide- and protamine sulphate-induced cystitis [732]. A hyperosmolar model of OAB has been claimed to be less invasive and more physiological compared to the cyclophosphamide model [349].
Bradykinin 2, but not bradykinin 1, receptors are expressed in rat bladder urothelium that mediate release of ATP. However, following acute (24 h) and chronic (8 day) cyclophosphamide-induced cystitis, bradykinin 1 receptor mRNA was detected throughout the bladder and this receptor also mediated ATP release and increase in [Ca2+]i [152]. Intravesical bradykinin activated the micturition pathway, which was attenuated by the P2 receptor antagonist PPADS.
Haemorrhagic cystitis is an adverse effect of therapy with cyclophosphamide used for the treatment of tumours and autoimmune conditions. P2X7 receptors, probably expressed by macrophages and neutrophils in the bladder submucosa, are increased in cyclophosphamide-induced haemorrhagic cystitis in mice [468]. Further, treatment with the P2X7 receptor antagonist, A-438079, or genetic ablation of this receptor reduced nociceptive behaviour and reduced oedema and haemorrhage. Epigallocatechin gallate, used to treat bladder cancer, attenuates IC in human bladder urothelium cells apparently by modulating purinergic receptors [439].
Bladder outflow obstruction
The expression of P2X1 receptors in bladder smooth muscle increased considerably in the symptomatically obstructed human bladder [81, 537]. In human obstructed bladders, activation of P2X1 receptors facilitated evoked release of ACh [614]. Myocytes from patients with bladder outflow obstruction from benign prostatic hypertrophy increased contractile responses to ATP relative to patients with OAB symptoms [65].
In animal models, the results of outlet obstruction have been reported. For example, in vivo release of ATP from urothelial cells in a rat model of bladder outlet obstruction was increased compared to controls [12]. In a rabbit model of bladder outflow obstruction there appeared to be a reduction of both atropine-sensitive and atropine-resistant responses, suggesting nerve damage [280]. However, when the contribution of cholinergic and purinergic neurotransmission to micturition contractions and bladder hyperactivity was investigated by continuous cystometry in unanaesthetised rats with outlet obstruction [327], it was concluded that both cholinergic and purinergic transmission are important for pressure generation and emptying of the bladder [327, 698]. The effects of purinergic receptor agonists were examined on hypertrophied smooth muscle of rat bladder, induced by partial ligation of the urethra giving an increase in bladder weight from 65 mg to 300 mg [622]. The force of contraction produced by ATP and α,β-meATP was significantly lower than in controls, and the rate of contraction slower. The contractile responses to ATP were attenuated in obstructed rat urinary bladder; this was shown to be due to a lowered rate of Ca2+ influx and maximal peak Ca2+ concentration, rather than to a decrease in P2X1 receptor density [621]. An increased population of ICCs in guinea-pig bladder following outlet obstruction has been reported [389]. P2X3 receptor up-regulation in ICCs in an experimental rat model of partial bladder outflow obstruction has been reported [427]. The sympathetic innervation of the bladder neck appears to be diminished in patients with bladder outlet obstruction [546], perhaps indicating that in this condition there is a reduced role for sympathetic nerve-released ATP as well as NA.
Suramin, a P2 receptor antagonist, enhanced the inhibitory action of atropine in detrusor from rats with outlet obstruction [562]. The authors suggest that this might be of interest in the therapy of patients with bladder incontinence caused by detrusor overactivity, who fail to respond to even the maximum dosage of antimuscarinic drugs.
Partial bladder outlet obstruction in the pig was characterised by reduced contractile responses to electrical field stimulation and to both muscarinic and purinergic agonists [488]. However, in the rat bladder model of partial outlet obstruction, ATP-induced contractions were significantly increased after 2 weeks and 3 months [509].
Botulinum toxin and ATP release
BTXA is being used increasingly for the treatment of detrusor overactivity (see [34, 159]). An early paper showed that BTXA inhibited release of ATP as well as ACh from parasympathetic nerves in the rat bladder [455] and confirmed subsequently [410, 654]. More recent studies have shown that BTX also inhibited ATP release from urothelium ([133, 329, 368, 626]; and see [177]). Daytime frequency, nocturia and pain were decreased by 44 %, 45 % and 79 %, respectively, by injection of BTXA unto the human bladder [625] and also urgency sensation as well as pain [410]. Bladder wall injection of BTXA in rat bladder after spinal cord injury reduces ATP release, while simultaneously increasing NO release from urothelium [624]. The authors hypothesise that alterations in the ratio of excitatory (ATP) and inhibitory (NO) urothelial transmitters promote bladder hyperactivity after spinal cord injury that can be reversed to a large extent with BTX. Intravesical infusion of either ATP or capsaicin can induce detrusor overactivity; BTX was more effective in blocking the effect of ATP than of capsaicin [42]. It was suggested that BTX could be used to treat OAB syndrome and bladder hypersensory states, especially those that may be caused by an augmentation of the purinergic pathway. BTXA is effective in the treatment of intractable detrusor overactivity; decreased levels of sensory receptors P2X3 and/or TRPV1 may contribute to its clinical effect [35, 42].
Synergistic stimulation of CGRP release from afferent nerve terminals by ATP and capsaicin has been claimed to be inhibited by BTXA [573]. BTXA injections into the trigone to treat idiopathic OAB do not induce vesicoureteral reflux [355]. Intraprostatic BTXA injections reduce prostate volume and thereby contribute to the recovery of spontaneous micturition in patients with chronic urinary retention [613].
Multiple sclerosis
MS patients often have peripheral symptoms, including bladder dysfunction [67, 68, 489] and it has been claimed that peripheral nerve damage occurs in the MS bladder [275]. Mice infected with the Semliki Forest Virus have been proposed as a model for the demyelinating disease, MS [716]. This model was used to study purinergic and cholinergic neurotransmission in the mouse bladder [502]. A selective change in purinergic transmission occurred in infected mice, while cholinergic transmission remained unchanged. There was a significant increase in the contractile responses to β,γ-meATP and in the purinergic (atropine-resistant) component of nerve-mediated contractions. The question was raised as to whether the increase in purinergic signalling is secondary to the bladder hypertrophy that occurs in this model or whether it is a primary event.
Bladder hyperactivity and incontinence in MS patients seems to be mediated in part by the emergence of involuntary bladder contractions induced by C-fibre bladder afferents. Desensitisation of the C-fibre afferents by intravesicle administration of afferent neurotoxins (capsaicin or resiniferatoxin) increases bladder capacity and reduces the number of incontinence episodes [132, 242, 244]. A role for purinergic mechanisms in the activation of bladder C-fibre afferents in MS patients has yet to be established.
Post-irradiation bladder dysfunction
Most interpretations of late irradiation injury of the urinary bladder have focussed on urothelial damage and fibrosis (see [272, 637]). In a study of rat detrusor strips taken 6 months after bladder X-irradiation at doses of 15 and 25 Gy, there was an increase in sensitivity to the purinergic agonist α,β-meATP, but no changes in the sensitivity to ACh or NA [693]. This led the authors to suggest that purinergic hypersensitivity in irradiated bladder, coupled with ultrastructural evidence of neural injury, leads to denervation supersensitivity that may contribute to the pathophysiology of post-irradiation bladder dysfunction.
Ischaemic bladder
Rabbit detrusor contractions elicited by nerve stimulation were more sensitive to bilateral ischaemia (3, 6 or 18 h duration) than were contractions to carbachol and ATP, indicating ischaemic damage to nerves [89]. This prejunctional change is consistent with previous studies of bladder outlet obstruction and ischaemia [279, 611].
Chronic alcohol consumption and bladder function
Amongst the many adverse effects of chronic alcohol consumption, autonomic neuropathies, affecting both sympathetic and parasympathetic system, are very common [347]. In a study of the effects of chronic (12 weeks duration) ethanol consumption in rats on bladder activity, it was found that neurally-evoked contractions and contractile responses to both carbachol and β,γ-meATP were potentiated. Cholinergic responses were more sensitive to ethanol than the purinergic responses, which showed limited potentiation at higher stimulation frequency and concentrations [380]. Chronic ethanol consumption impairs purinoceptor- as well as adrenoceptor-mediated relaxation of isolated rat detrusor smooth muscle [125, 688]. Ethanol has been shown to alter the neuronal P2X receptor so that the ATP concentration–response curve is shifted to the right, which involves an allosteric action to decrease agonist affinity [426].
Vitamin E deficiency
Vitamin E (α-tocopherol) is essential for normal neuronal physiology and its deficiency results in neuropathic changes (see [503]). The effects of vitamin E deficiency were studied on neuromuscular transmission in the caecum, vas deferens and urinary bladder of the rat [316]. While both pre- and postjunctional dysfunctions were produced in the caecum, no changes in sympathetic neuromuscular transmission were observed in the vas deferens or in parasympathetic neuromuscular transmission in the bladder.
Diabetes
Disturbances in micturition and damage to autonomic nerves supplying the human urinary bladder in diabetes has been known for many years (see [21, 43, 45, 84, 229, 235, 245,246, 469]). The major clinical feature of diabetic bladder dysfunction is a gradual loss of bladder sensation and motor function, resulting in a large bladder and chronic residual urine volume. Similar changes have been identified in the streptozotocin (STZ)-induced diabetic rat model (see [29, 382, 390, 432, 444, 635]), in the diabetic Chinese hamster [184], and in most reports of the alloxan-induced diabetic rat model [547, 548, 569, 677, 689]. Functional abnormalities associated with progressive axonopathy of afferent myelinated sensory nerve fibres and later of unmyelinated efferent preganglionic fibres were also described in the spontaneously diabetic BioBreeding rat [549]. In urethral rings from STZ diabetic rats, the contractile responses to field stimulation, ACh and NA were unchanged compared to controls [458]. It has been claimed that in the rat urinary bladder, STZ diabetes causes impairment of capsaicin-sensitive sensory fibres even at 4 weeks, but not of the cholinergic system, and also stimulates the release of epithelial contracting factors, and further that epithelium removal impairs ACh-induced contractions in diabetic bladder, but not in controls [558]. ATP released from the urothelium has been reported to contribute to bladder dysfunction in type 2 diabetes [711].
An early study of contractions of the diabetic rat bladder showed reduced nerve-mediated responses, but only a tendency for reduced responses to ATP, ACh and KCl [441] (Fig. 7a and b). Although there was some indication of neuropathy of motor nerves in the STZ diabetic bladder [431], no sign of damage to capsaicin-sensitive sensory nerves in the bladder was observed, at least in 7- to 9-week-old diabetic animals [595]. A transient increase in sensitivity of the 6- and 12-week-old STZ rat whole bladder preparations to α,β-meATP was reported [501]; the biphasic response of the bladder to α,β-meATP was not changed significantly in earlier 4- to 5-week-old STZ rats [391]. α,β-MeATP caused increase in spontaneous bladder activity in STZ diabetic rats and responses to nerve stimulation were greater than controls, including the component mediated by P2X receptors [657]. In another study of bladder strips taken from STZ rats, it was shown that atropine-resistant (purinergic) responses to field stimulation were reduced and it was concluded that this was probably the result of a reduction in release of the NANC transmitter [447]. The same authors found a potentiation of cholinergic transmission in the STZ bladder, and suggested this was due to enhanced release of ACh [448]. More recent papers, however, report that while the cholinergic component of the nerve stimulated contraction is diminished in the STZ rat and diabetic rabbit bladders, the purinergic component is enhanced [433, 504].
a and b Responses of bladder body strips from streptozotocin-diabetic rats. a Responses to nerve stimulation (supramaximal voltage, 0.5 ms pulse duration for 2 s every 2 min) of bladder strips from 2-month-old streptozotocin-diabetic (filled triangle, n = 5) and control rats (filled circle, n = 5). (b) Responses to ATP of bladder strips from 2-month-old streptozotocin-diabetic (filled triangle, n = 5) and control rats (filled circle, n = 5). For both graphs, each point represents the mean ± SEM and data are expressed as grams tension per 100 mg tissue. (a and b Reproduced from [441], with permission from ASEPT.) c Spontaneous and distension-induced activity in ureter afferent fibres. Multifibre afferent responses to rapid distension. Note that background afferent activity occurs in bursts and that ureter distension results in an initial burst of discharge (circle) followed by a phase of maintained activity (bar). d ATP can sensitise ureter afferent fibres. An example representative of distension-induced afferent activity before and following intraluminal application of increasing concentrations of ATP. (c and d Reproduced from [579], with permission from Elsevier.) e ATP concentration ([ATP]) in perfusate immediately before and after distension of the human ureter, grouped in pressure ranges. The mean [ATP] after distension is significantly greater than before distension in each pressure range (**P < 0.01; n ≥ 7 for each group of distensions, error bars represent SEM). (Reproduced from [126], with permission from Springer
The calcium channel blocker, nifedipine, has been shown to block the purinergic component of the parasympathetic contractile responses of the bladder [70]. No significant differences were found in the sensitivity of bladder strips from control and STZ diabetic rats to antagonism by nifedipine [443]. ATP significantly increases the endogenous release of PGF2 and PGF2α from the urothelium of 4-week-old STZ diabetic rat bladder and it was proposed that P2X receptors are present on urothelial cells as well as smooth muscle [565]. Increased synthesis of prostanoids during epithelial irritation may produce hyperactivity or spasm of the detrusor muscle [309].
In contrast to normal mouse bladder, the urinary bladder of STZ mice had weaker neurogenic contractile responses to electrical field stimulation [436]. Nerve-mediated contractions in diabetic bladders were also less sensitive to the depressant actions of uranyl nitrate, which was shown in the same study to be selective for the non-cholinergic contractile component. Since high Ca2+ or calmodulin inhibitors antagonised the suppressant effect of uranyl nitrate, it was postulated that Ca2+ regulation of ATP release might be impaired in the diabetic state.
Bladders from 8-week-old STZ diabetic rats showed enhanced relaxant responses to ATP and adenosine, as well as increased contractile responses to ATP [276]. Enhanced responses to ATP, but not to ACh or KCl, were also reported in the 4-week-old STZ diabetic bladder, and it was also shown that the responses of detrusor muscle from diabetic ovariectomized rats were decreased, although partially recovered to control values by oestrogen treatment [564]. A further paper showed that contractile responses of bladder from STZ-diabetic rats to ATP and nerve stimulation peaked at 6 to 9 weeks, but reverted to those of control by 12–20 weeks [188]. Purinergic and cholinergic receptor activation triggers a significantly greater release of ATP, but not NO, in STZ female rat bladders [506]. There is an upregulation of muscarinic M3 and P2X1 receptors in the early phase of STZ diabetic bladder, but downregulation of the P2X2 receptor [435]. Bladder overactivity that developed 2 months after STZ-induced diabetes was accompanied by significant increase in expression of P2Y2 and P2Y4 receptors in the bladder [639]. Diabetes and metabolic syndrome are known risk factors for the development of lower urinary tract symptoms, including OAB. Using a long-term fructose-induced metabolic syndrome model, upregulation of postjunctional purinergic and muscarinic cholinergic receptor expression was demonstrated [155].
Goshe-jinki-gan, a traditional Chinese herbal mixture thought to affect sensory nerves, has been used to treat patients with dysuria due to diabetes (as well as for patients with urinary incontinence). This medicine is claimed to decrease detrusor contractions and increase bladder capacity, while not reducing voiding pressure. It has been reported that goshe-jinki-gan reduces urothelial P2X3 receptors without destroying the nerve fibres [331].
Bladder cancer
ATP reduces the growth of high grade bladder cancer cells, both in vitro and in vivo ([39, 606]; see also [605]). Doxorubicin, used for the treatment of superficial bladder cancer, has significant side effects, including dysuria, increased urinary frequency and urgency, and it has been shown to inhibit stretch-stimulated release of ATP from urothelial cells [148], as well as induction of inflammatory cytokines and enhanced release of PGE2 [354]. Quercetin, a plant-derived flavonoid, has been used to prevent bladder cancer in cells lines, by inhibiting cell proliferation, promoting cell cycle arrest or cell death by inhibiting ecto-nucleotidase activity [452, 578].
Benign prostatic hyperplasia
Urothelial cells from patients with benign prostatic hyperplasia release significantly more ATP in response to stretch than control urothelial cells [649]. The authors also showed that the α1-adrenoceptor antagonist doxazosin, which has been used to ameliorate benign prostatic hyperplasia-inducing hypersensory voiding symptoms, inhibited the ATP release.
Enterocytoplasty bladders
Incorporation of bowel tissue into the bladder wall has been used to increase bladder capacity or decrease bladder pressure (see [603]). It is possible in vitro to differentiate pharmacologically between intestinal and detrusor muscle by studying the response to ATP, which is contractile in detrusor, but relaxant in intestinal muscle [49]. In rabbit, intestinal muscle dissected from ileocystoplasties showed after 4 weeks to 3 months a contractile response to nerve stimulation and ATP [48, 265].
Bacterial infection
Bacterial infection of the bladder can lead to overactivity and urinary incontinence and urgency and it has been shown that there is an increase in ATP in intravesicular fluid in this condition [146]. Treatment of urothelial cells with lipopolysaccharide from enteropathogenic Escherichia coli reduced stretch-induced ATP release, suggesting that infection may alter urothelial purinergic signalling in response to filling [464]. Pseudomonas aeruginosa is a bacterium responsible for many hospital acquired urinary tract infections. P. aeruginosa leads to the production of pyocyanin, a virulence factor that reduces ATP release from urothelial cells [478]. In this respect, it is interesting that ATP concentrations in the urine were lower during episodes of bacteriuria [709]. ATP induces IL-8 and IL-6 release from the human renal epithelial cell line A498 via P2Y2 receptors and it was suggested that this may be important for neutrophil recruitment and function in uropathogenic E. coli infected urothelium [388]. A test for bacteriuria in urine has employed an ATP assay method for many years [451] and is a rapid and reliable diagnostic tool. Urinary tract infections cause complications for renal transplant recipients and it has been suggested that urinary ATP and bacteria in urothelial cells shed from the bladder is a superior diagnostic marker for urinary tract infections in renal transplant recipients [363].
Urethra
In many species, including rabbit, cat and humans, there is a NANC inhibitory transmission to the urethra [30, 295, 338, 378, 474, 480, 619]. Amongst compounds which cause relaxation, the putative transmitter was claimed not to be VIP, ATP, 5-HT or adenosine, because blockade of these responses by pharmacological manipulation did not produce a parallel effect on the neurogenic response [282, 295, 377, 720]. The principal NANC inhibitory transmitter is now clearly established, namely, NO [26, 214, 258, 283, 414, 563, 655, 674]. However, a small component of purinergic neurotransmission may also be involved [24, 561, 563]. ATP has been shown to cause urethral relaxation, perhaps via P2Y1 receptors, in pigs [719], guinea-pigs [124], rabbits [533] and hamsters [559]. It is interesting that ATP has been claimed to be an inhibitory neurotransmitter to the guinea-pig urinary bladder neck [294]. When the tone of the urethra is raised, ATP caused relaxation, but if the tone of the urethra is low, high concentrations of ATP can cause contraction [124, 169, 351, 553]. A more recent paper has claimed that the excitatory effect of ATP on rabbit urethral smooth muscle is mediated by activation of P2Y receptors on ICCs, which act as pacemakers [85]. Bursts of spikes in the urethra were initiated by NA or ACh, but inhibited by ATP [124], perhaps after breaking down to adenosine. In the pre-contracted proximal urethra of the hamster, NANC nerve stimulation and exogenous ATP were also shown to produce relaxations, which were attenuated by suramin and Reactive Blue 2, and to a lesser extent by 8-PT, but not by PPADS. ATP-induced relaxations were also reduced by indomethacin and were urothelium- and NO-independent, since they were not affected by removal of the urothelium or by the NOS inhibitor N ω-nitro-l-arginine methyl ester [561]. Thus, P2Y as well as P1 receptors appear to mediate the relaxing effect of ATP released from a NANC nerve pathway which has a subordinate role to the major nitrergic pathway. In a study of the roles of purines in neurally mediated urethral relaxation in male rabbits, NANC relaxations were shown to be reduced by suramin, as well as by the NOS inhibitor l-NOARG, and in superfusion experiments electrical field stimulation markedly increased the outflow of ATP into the superfusate. It was suggested that P2Y receptors exist in male rabbit urethra and that ATP and related compounds may play a role in NANC transmission [533]. In a microelectrode study, transmural stimulation of longitudinal smooth muscle strips from guinea-pig urethra evoked EJPs and triggered slow waves that were abolished by α,β-meATP as well as by tetrodotoxin [286]. The authors concluded that stimulation of purinoceptors by neurally released ATP initiates EJPs in the guinea-pig urethra and also causes the release of Ca2+ from intracellular stores to evoke slow waves. Evidence has been presented that purinergic neurotransmission to rabbit urethral muscle produces contraction via activation of P2X receptors on smooth muscle cells [86]. Since the responses were blocked by α,β-meATP, the P2X1 receptor subtype is likely to be involved.
Sympathetic (hypogastric) nerve stimulation produced a contraction of the urethra, which was significantly reduced by quinidine [165], suggestive of sympathetic purinergic cotransmission. The responses to other sympathetic efferent pathways projecting to the urethra [369] have not been examined. ATP may also be released from sensory nerve fibres supplying the urethra during axon reflex activity (see [102]).
PGs, which can be produced following occupation of P2Y receptors, may play an important role in bladder or urethral contractility in physiological or pathophysiological conditions. For example, in the rat, SC19220 reduced detrusor tone resulting in an increased bladder capacity and decrease in voiding efficiency [460]. Similarly, treatment with indomethacin caused an increase in the residual volume of the bladder at the end of micturition [458]. Vesical distension in dogs causes a reflex decrease in urethral resistance, accompanied by a large increase in PGE2 in urethral venous outflow. The reflex inhibition and release of PGE2 are prevented when ganglionic transmission is blocked by pentolinium [261]. These results imply that a physiological mechanism stimulates PGE2 synthesis in the urethra, and that endogenous PGs are important in maintaining tone in the smooth muscle, as shown by indomethacin or SC19220 causing relaxation of isolated preparations [338].
Sensory innervation of the urethra is conveyed to the spinal cord mainly via the pelvic nerves and DRG and, to some extent, via the hypogastric nerve (see [430]). Sensory function in the urethra is via afferent fibres that express P2X3 receptors [127]. High threshold afferent C-fibres might be related to nociception, while low threshold fibres initiate a usually inactive, non-voluntary spinal micturition reflex [365].
Ureter
Functional expression of purinoceptors
The innervation of the ureter is sparse, perhaps because peristaltic activity is myogenic rather than neurogenic as in the gut. The dominant nerve components are sensory nerves, largely confined to a suburothelial plexus [307]. These afferent nerves might play a role during vesicoureteral reflux which could distend the ureter and activate reflexes that modulate urine delivery to the bladder [666]. Pelvic nerve stimulation produced a modest, transient decrease in ureteral peristaltic frequency, while hypogastric nerve stimulation produced different responses depending on the detrusor pressure.
The first evidence presented for the involvement of purines as neurotransmitters or neuromodulators in vesicoureteral reflex activity was described for the cat ureter [666]. ATP was shown to constrict the pig ureter, while intravesical adenosine caused relaxation via A2B receptors on smooth muscle and may modulate the ureteral NANC excitatory transmission through a postjunctional mechanism [293]. ATP, α,β-meATP and adenosine produced transient decreases in ureteral peristaltic frequency and in the spontaneous firing of the renal nerve. Theophylline blocked the effect of adenosine, but not ATP, so both P1 and P2 receptors are likely to be involved. P2 purinoceptors were first identified immunohistochemically in the ureter by Lee et al. [412]. The authors showed expression of P2X1 receptors on smooth muscle membranes, P2X5 and probably P2X7 receptors on uroepithelium and P2X6 receptors in the layer beneath the urothelium of the rat ureter. P2X3 receptors were shown to be localised on subepithelial sensory nerves. In addition P2X1, P2X2, P2X4 and P2X7 receptors were localised on the smooth muscle of blood vessels in the rat ureter and by analogy with other visceral blood vessels it is likely that P2Y and P2X4 receptors are expressed by vascular endothelial cells [121]. It is likely that some of the purinoceptors present in the ureter participate in long-term (trophic) events during development and regeneration, such as cell proliferation, migration, differentiation and cell death (see [2]).
Renal colic
The electrical activity in mechanosensitive C-fibre afferent units was recorded in small ureteric branches of the hypogastric nerve during various mechanical stimuli, including probing of the ureter with small glass probes, insertion of an intraluminal glass bead to mimic kidney stones and distension of the ureter using hydrostatic pressure [131,592]. It was suggested that some of these afferent fibres might be involved in the signalling of nociceptive events. In the later paper from this group, they distinguished two classes of mechanosensitive afferent fibres in the guinea-pig ureter: U1 units monitoring normal peristalsis and U2 units perhaps involved in the signalling of noxious events. A third class of mechanosensitive units was identified in the chicken ureter [277]. Units responding to peristaltic movements of the ureter have also been reported by another group, who suggested that one of the functions of ureteric afferents might be the monitoring of peristaltic rhythms [341]. Evidence has been presented that spinothalamic tract neurones mediate nociceptive responses to ureteral occlusion [20]. Recordings from dorsal horn neurones in the spinal cord (T12–L1) in anaesthetised rats led to the conclusion that they receive both noxious and innocuous ureter stimulation mainly from high-threshold afferents and their response properties correlate well with ureteric pain sensations in humans [404]. In a later paper from this group, spinal neurone recording after implantation of an experimental ureteric stone led to the conclusion that the presence of ureteric stone evokes excitability changes in spinal neurones (enhanced background activity, a greater number of ureter-driven cells, decreased threshold of convergent somatic receptor fields) which likely account for the referred hyperalgesia seen in rats with calculosis [581].
Burnstock [104] proposed that in tubes (e.g., ureter, salivary duct, bile duct, vagina and intestine) and in sacs (e.g., urinary bladder, gall bladder and lung), nociceptive mechanosensory transduction occurs where distension releases ATP from the epithelial cells lining these organs, which then activates P2X3 and/or P2X2/3 receptors on subepithelial sensory nerve plexuses to relay messages to the CNS pain centres (see [105]).
In a later study, it was shown that distension of the guinea-pig ureter increased spike discharge in sensory neurons, which was mimicked by ATP and reduced by ATP antagonists [579] (Fig. 7c and d). The afferent responses consisted of both fast and slow components. The P2 receptor antagonists TNP-ATP and PPADS reduced distension-induced afferent activity and blocked the rapid and reduced the slower response to ATP, while the remaining responses were blocked by the selective A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine. The ecto-ATPase inhibitor (ARL-67156) produced an increase in baseline and distension-induced sensory discharge.
Knight et al. [379] found that distending the perfused guinea-pig ureter at pressures from 20 to 700 cm H2O caused a pressure-dependent release of ATP from urothelial cells, approximately ten times the basal release levels. The ATP release was abolished by removal of the urothelium and scanning electron microscopy confirmed an intact urothelium after distension. ATP was not released due to activation of stretch-activated channels since gadolinium failed to affect ATP release, nor did glibenclamide, known to inhibit ABC proteins. However, both monensin and brefeldin A, which interfere with vesicular formation and trafficking, inhibited distension-evoked ATP release, which was Ca2+-dependent, indicating that ATP release from ureter urothelium might be largely mediated by vesicular exocytosis.
In a study in our laboratory, experiments have been carried out to show that ATP is released from the human ureter upon distension (Fig. 7e) and that human ureteric suburothelial sensory nerves express P2X3 receptors [126]. The release of ATP only occurred above a threshold of 25–30 cm H2O. This is similar to the uroteric pressure threshold for pain measured by Risholm [576]. In a review of the physiology and pharmacology of the human ureter, it was suggested that purinergic receptors might be target analgesics for the treatment of ureteral colicky pain and that an additional advantage might be facilitating spontaneous ureteral stone passage [128].
P2X7 receptors and ureteral inflammation and interstitial fibrosis
A study has been carried out to investigate the role of P2X7 receptors in the inflammatory and fibrogenic responses of the kidneys to unilateral ureteral obstruction (UUO) by using P2X7 KO mice [271]. It was shown that 7 days after UUO in WT mice there was increased expression of P2X7 receptors associated with inflammation and fibrogenic responses in the cortex, although no positive cells were detected in the interstitium. However, no P2X7 receptor immunopositivity was seen after 14 days. P2X7 receptor KO mice did not exhibit the alterations seen in the WT mice. There were less macrophages in the interstitium, a lower population of myofibroblasts, diminished collagen deposition, as well as decreased transforming growth factor β expression in the renal interstitium and less apoptotic cells. The authors suggest that there is a potential role for P2X7 receptor antagonists to prevent renal interstitial fibrosis. A2A receptor KO significantly increased the progression of renal interstitial fibrosis in a mouse model of UUO [731].
Concluding comments
Despite earlier scepticism, there is now abundant evidence for purinergic signalling in the lower urinary tract, in particular parasympathetic purinergic contractions of urinary bladder (via P2X1 receptors) and P2X3 receptors on sensory nerve terminals involved in the micturition reflex and pain. Purinergic synaptic transmission occurs in pelvic ganglia and brain stem regions controlling bladder activities.
Of special interest is the evidence for enhanced purinergic signalling in pathological conditions such as obstructive bladder and BPS/IC encouraging the future development of purinergic therapeutic drugs that hopefully will be developed and bring benefit and relief to many patients with urinary tract disorders.
References
Abbracchio MP, Burnstock G (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64:445–475
Abbracchio MP, Burnstock G (1998) Purinergic signalling: pathophysiological roles. Jpn J Pharmacol 78:113–145
Abrams P (2003) Describing bladder storage function: overactive bladder syndrome and detrusor overactivity. Urology 62:28–37
Acevedo CG, Contreras E (1985) Possible involvement of adenine nucleotides in the neurotransmission of the mouse urinary bladder. Comp Biochem Physiol C 82:357–361
Acevedo CG, Contreras E (1989) Effect of extracellular calcium and calcium channel antagonists on ATP and field stimulation induced contractions of the mouse urinary bladder. Gen Pharmacol 20:811–815
Acevedo CG, Contreras E, Escalona J, Lewin J, Huidobro-Toro JP (1992) Pharmacological characterization of adenosine A1 and A2 receptors in the bladder: evidence for a modulatory adenosine tone regulating non-adrenergic non-cholinergic neurotransmission. Br J Pharmacol 107:120–126
Acevedo CG, Lewin J, Contreras E, Huidobro-Toro JP (1990) Bradykinin facilitates the purinergic motor component of the rat bladder neurotransmission. Neurosci Lett 113:227–232
Ahluwalia A, Giuliani S, Scotland R, Maggi CA (1998) Ovalbumin-induced neurogenic inflammation in the bladder of sensitized rats. Br J Pharmacol 124:190–196
Aizawa N, Igawa Y, Andersson KE, Iijima K, Nishizawa O, Wyndaele JJ (2011) Effects of intravesical instillation of ATP on rat bladder primary afferent activity and its relationship with capsaicin-sensitivity. Neurourol Urodyn 30:163–168
Aizawa N, Wyndaele JJ, Homma Y, Igawa Y (2012) Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn 31:148–155
Akasu T, Shinnick-Gallagher P, Gallagher JP (1984) Adenosine mediates a slow hyperpolarizing synaptic potential in autonomic neurones. Nature 311:62–65
Akino H, Nagase K, Watanabe N, Tanase K, Oyama N, Miwa Y, Yokoyama O (2011) ATP release from bladders is increased in-vivo and suppressed by alpha-1 adrenoceptor blocker in a rat model of bladder outlet obstruction. Eur Urol Suppl 10:303–304
Albino-Teixeira A, Azevedo I, Branco D, Osswald W (1990) Purine agonists prevent trophic changes caused by sympathetic denervation. Eur J Pharmacol 179:141–149
Alkondon M, Ganguly DK (1980) Release of prostaglandin E from the isolated urinary bladder of the guinea-pig. Br J Pharmacol 69:573–577
Alm P (1978) Cholinergic innervation of the human urethra and urinary bladder: a histochemical study & review of methodology. Acta Pharmacol Toxicol 43:56–62
Alm P, Elmér M (1975) Adrenergic and cholinergic innervation of the rat urinary bladder. Acta Physiol Scand 94:36–45
Alm P, Elmér M (1979) Adrenergic reinnervation of the denervated rat urinary bladder. Experientia 35:1387–1388
Ambache N, Killick SW, Woodley JP (1977) Evidence against purinergic motor transmission in guinea-pig urinary bladder. Br J Pharmacol 61:464P
Ambache N, Zar MA (1970) Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol 210:761–783
Ammons WS (1989) Primate spinothalamic cell response to ureteral occlusion. Brain Res 496:124–130
Andersen JT, Bradley WE (1976) Abnormalities of bladder innervation in diabetes mellitus. Urology 7:442–448
Anderson GF (1982) Evidence for a prostaglandin link in the purinergic activation of rabbit bladder smooth muscle. J Pharmacol Exp Ther 220:347–352
Andersson K-E (1993) Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev 45:253–308
Andersson KE (2001) Neurotransmission and drug effects in urethral smooth muscle. Scand J Urol Nephrol Suppl 207:26–34
Andersson KE (2002) Bladder activation: afferent mechanisms. Urology 59:43–50
Andersson K-E, Garcia-Pascual A, Forman A, Tøttrup A (1991) Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand 141:133–134
Andersson KE, Hedlund P (2002) Pharmacologic perspective on the physiology of the lower urinary tract. Urology 60:13–20
Andersson K-E, Husted S, Sjögren C (1980) Contribution of prostaglandins to the adenosine triphosphate-induced contraction of rabbit urinary bladder. Br J Pharmacol 70:443–452
Andersson PO, Malmgren A, Uvelius B (1988) Cystometrical and in vitro evaluation of urinary bladder function in rats with streptozotocin-induced diabetes. J Urol 139:1359–1362
Andersson K-E, Mattiasson A, Sjögren C (1983) Electrically induced relaxation of the noradrenaline contracted isolated urethra from rabbit and man. J Urol 129:210–214
Andersson K-E, Sjögren C (1982) Aspects on the physiology and pharmacology of the bladder and urethra. Prog Neurobiol 19:71–89
Apodaca G (2004) The uroepithelium: not just a passive barrier. Traffic 5:117–128
Apodaca G, Balestreire E, Birder LA (2007) The uroepithelial-associated sensory web. Kidney Int 72:1057–1064
Apostolidis A, Dasgupta P, Fowler CJ (2006) Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 49:644–650
Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P (2005) Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 174:977–982
Araki I, Du S, Kobayashi H, Sawada N, Mochizuki T, Zakoji H, Takeda M (2008) Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol 15:681–687
Aronsson P, Andersson M, Ericsson T, Giglio D (2010) Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 107:603–613
Aronsson P, Johnsson M, Vesela R, Winder M, Tobin G (2012) Adenosine receptor antagonism suppresses functional and histological inflammatory changes in the rat urinary bladder. Auton Neurosci 171:49–57
Artim DE, Birder LA, de Groat WC (2007) Purinergic mechanisms in human bladder cancer cells. FASEB J 21:A1349–A134b
Atala A, Vacanti JP, Peters CA, Mandell J, Retik AB, Freeman MR (1992) Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J Urol 148:658–662
Athanasopoulos A, Cruz F (2011) The medical treatment of overactive bladder, including current and future treatments. Expert Opin Pharmacother 12:1041–1055
Atiemo H, Wynes J, Chuo J, Nipkow L, Sklar GN, Chai TC (2005) Effect of botulinum toxin on detrusor overactivity induced by intravesical adenosine triphosphate and capsaicin in a rat model. Urology 65:622–626
Barkai L, Szabo L (1993) Urinary bladder dysfunction in diabetic children with and without subclinical cardiovascular autonomic neuropathy. Eur J Pediatr 152:190–192
Barras M, Van der Graaf PH, Angel I (1996) Characterisation of the 5-HT receptor potentiating neurotransmission in rabbit bladder. Eur J Pharmacol 318:425–428
Bartley O, Brolin I, Fagerberg SE, Wilhelmsen L (1966) Neurogenic disorders of the bladder in diabetes mellitus. A clinical–roentgenological investigation. Acta Med Scand 180:187–198
Batra S (1979) Subcellular distribution and cytosolic receptors of progesterone and of estradiol-17β in the rabbit myometrium: effect of progesterone treatment. Biol Reprod 21:483–489
Batra S (1980) Estrogen and smooth muscle function. Trends Pharmacol Sci 1:388
Batra AK, Hanno PM, Ruggieri MR (1992) Detubularization-induced contractile response change of the ileum following ileocystoplasty. J Urol 148:195–199
Batra AK, Wein AJ, Ruggieri MR, Levin RM (1987) Comparative response of smooth muscle strips of bladder and bowel to various pharmacological agents. Neurourol Urodyn 6:351–357
Bayliss M, Wu C, Newgreen D, Mundy AR, Fry CH (1999) A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J Urol 162:1833–1839
Beckel JM, Birder LA (2012) Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol 590:1465–1480
Belis JA, Colby JE, Adlestein LB, Westfall DP (1981) Characterization of neurotransmission in the bladder using arylazido-amino-propionyl ATP (ANAPP3). Am Coll Surg XXXII:625–627
Bhat MB, Mishra SK, Raviprakash V (1989) Sources of calcium for ATP-induced contractions in rat urinary bladder smooth muscle. Eur J Pharmacol 164:163–166
Bhat MB, Mishra SK, Raviprakash V (1989) Differential susceptibility of cholinergic and noncholinergic neurogenic responses to calcium channel blockers and low Ca2+ medium in rat urinary bladder. Br J Pharmacol 96:837–842
Birder LA (2005) More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol 289:F489–F495
Birder LA (2006) Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol 45:221–226
Birder LA, Andersson KE (2013) Urothelial signalling. Physiol Rev 93:653–680
Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA (2003) Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 285:F423–F429
Birder LA, de Groat WC (2007) Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4:46–54
Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M (2010) Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn 29:128–139
Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, Caterina M (2007) Activation of urothelial transient receptor potential vanilloid 4 by 4α-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323:227–235
Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, de Groat WC, Apodaca G, Watkins S, Caterina MJ (2002) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5:856–860
Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287:F1084–F1091
Birder LA, Ruggieri M, Takeda M, van Koeveringe G, Veltkamp S, Korstanje C, Parsons B, Fry CH (2012) How does the urothelium affect bladder function in health and disease? ICI-RS 2011. Neurourol Urodyn 31:293–299
Bishara S, Gao H, Malone-Lee M, Lunawat R, Khan S, Kelsey M, Khasriya R, King B, Malone-Lee J (2009) Increased ATP mediated contraction of isolated detrusor cells of patients with outflow obstruction. Neurourol Urodyn 28:746–747
Blackburn GM, Kent DE, Kolkmann F (1984) The synthesis and metal binding characteristics of novel isopolar phosphonate analogues of nucleotides. J Chem Soc Perkin Trans 1(1):1119–1125
Blaivas JG, Bhimani G, Labib KB (1979) Vesicourethral dysfunction in multiple sclerosis. J Urol 122:342–347
Blaivas JG, Holland NJ, Giesser B, LaRocca N, Madonna M, Scheinberg L (1984) Multiple sclerosis bladder. Studies and care. Ann N Y Acad Sci 436:328–346
Bo X, Burnstock G (1989) [3H]-α, β-methylene ATP, a radioligand labelling P2-purinoceptors. J Auton Nerv Syst 28:85–88
Bo X, Burnstock G (1990) The effects of Bay K8644 and nifedipine on the responses of rat urinary bladder to electrical field stimulation, β, γ-methylene ATP and acetylcholine. Br J Pharmacol 101:494–498
Bo X, Burnstock G (1990) High- and low-affinity binding sites for [3H]-α, β-methylene ATP in rat urinary bladder membranes. Br J Pharmacol 101:291–296
Bo X, Burnstock G (1992) Species differences in localization of [3H]α, β-methylene ATP binding sites in urinary bladder and urethra of rat, guinea-pig and rabbit. Eur J Pharmacol 216:59–66
Bo X, Burnstock G (1993) Triphosphate, the key structure of the ATP molecule responsible for interaction with P2X purinoceptors. Gen Pharmacol 24:637–640
Bo X, Burnstock G (1995) Characterization and autoradiographic localization of [3H]α, β-methylene adenosine 5′-triphosphate binding sites in human urinary bladder. Br J Urol 76:297–302
Bo X, Fischer B, Maillard M, Jacobson KA, Burnstock G (1994) Comparative studies on the affinities of ATP derivatives for P2X-purinoceptors in rat urinary bladder. Br J Pharmacol 112:1151–1159
Boland B, Himpens B, Paques C, Casteels R, Gillis JM (1993) ATP induced-relaxation in the mouse bladder smooth muscle. Br J Pharmacol 108:749–753
Bolego C, Abbracchio MP, Cattabeni F, Ruzza R, Puglisi L (1995) Effects of ADPβS and UTP on the rat urinary bladder smooth muscle. Res Comm Mol Pathol Pharmacol 87:75–76
Bolego C, Pinna C, Abbracchio MP, Cattabeni F, Puglisi L (1995) The biphasic response of rat vesical smooth muscle to ATP. Br J Pharmacol 114:1557–1562
Borvendeg SJ, Al Khrasani M, Rubini P, Fischer W, Allgaier C, Wirkner K, Himmel HM, Gillen C, Illes P (2003) Subsensitivity of P2X but not vanilloid 1 receptors in dorsal root ganglia of rats caused by cyclophosphamide cystitis. Eur J Pharmacol 474:71–75
Boselli C, Bianchi L, Grana E (1997) Effect of cromakalim on the purinergic and cholinergic transmission in the rat detrusor muscle. Eur J Pharmacol 335:23–30
Boselli C, Govoni S, Condino AM, D'Agostino G (2001) Bladder instability: a re-appraisal of classical experimental approaches and development of new therapeutic strategies. J Auton Pharmacol 21:219–229
Brading AF, Mostwin JL (1989) Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br J Pharmacol 98:1083–1090
Brading AF, Williams JH (1990) Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and α, β-methylene ATP. Br J Pharmacol 99:493–498
Bradley WE (1980) Diagnosis of urinary bladder dysfunction in diabetes mellitus. Ann Intern Med 92:323–326
Bradley E, Kadima S, Drumm B, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP (2010) Novel excitatory effects of adenosine triphosphate on contractile and pacemaker activity in rabbit urethral smooth muscle. J Urol 183:801–811
Bradley E, Kadima S, Kyle B, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP (2011) P2X receptor currents in smooth muscle cells contribute to nerve mediated contractions of rabbit urethral smooth muscle. J Urol 186:745–752
Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P (2004) P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol 46:247–253
Bramich NJ, Brading AF (1996) Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol 492:185–198
Bratslavsky G, Whitbeck C, Horan P, Levin RM (1999) Effects of in vivo ischemia on contractile responses of rabbit bladder to field stimulation, carbachol, ATP and KCl. Pharmacology 59:221–226
Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN (2006) β-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol 290:F486–F495
Brown AD (1977) Postmenopausal urinary problems. Clin Obstet Gynaecol 4:181–206
Brown CM, Burnstock G (1981) The structural conformation of the polyphosphate chain of the ATP molecule is critical for its promotion of prostaglandin biosynthesis. Eur J Pharmacol 69:81–86
Brown C, Burnstock G, Cocks T (1979) Effects of adenosine 5′-triphosphate (ATP) and β-γ-methylene ATP on the rat urinary bladder. Br J Pharmacol 65:97–102
Buchthal F, Kahlson G (1944) The motor effect of adenosine triphosphate and allied phosphorus compounds on smooth mammalian muscle. Acta Physiol Scand 8:325–334
Buck AC, McRae C, Reed PI, Chisholm GD (1974) The diabetic bladder. Proc Roy Soc Med 67:81–83
Buffington CA, Kiss S, Kanai AJ, Dineley K, Roppolo JR, Reynolds IR, de Groat WC, Birder LA (2000) Alterations in urothelium and bladder afferents in feline interstitial cystitis. Soc Neurosci Abstr 349:2
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Burnstock G (1976) Do some nerve cells release more than one transmitter? Neuroscience 1:239–248
Burnstock G (1978) A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L (eds) Cell membrane receptors for drugs and hormones: a multidisciplinary approach. Raven Press, New York, pp 107–118
Burnstock G (1990) Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochem Int 17:357–368
Burnstock G (1990) Co-transmission. The Fifth Heymans memorial lecture — Ghent, February 17, 1990. Arch Int Pharmacodyn Ther 304:7–33
Burnstock G (1993) Series editor. The autonomic nervous system. Volume 3. Nervous control of the urogenital system. Harwood Academic Publishers, Switzerland, pp 1–588
Burnstock G (1995) Noradrenaline and ATP: cotransmitters and neuromodulators. J Physiol Pharmacol 46:365–384
Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–342
Burnstock G (2001) Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22:182–188
Burnstock G (2001) Purinergic signalling in lower urinary tract. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology, volume 151/I. Purinergic and pyrimidinergic signalling I — molecular, nervous and urinogenitary system function. Springer-Verlag, Berlin, pp 423–515
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483
Burnstock G (2009) Purinergic cotransmission. Exp Physiol 94:20–24
Burnstock G (2011) Therapeutic potential of purinergic signalling for diseases of the urinary tract. BJU Int 107:192–204
Burnstock G (2013) Purinergic signalling in the lower urinary tract. Acta Physiol 207:40–52
Burnstock G, Allen TGJ, Hassall CJS, Pittam BS (1987) Properties of intramural neurones cultured from the heart and bladder. In: Heym C (ed) Histochemistry and cell biology of autonomic neurons and paraganglia. Exp. Brain Res. Ser. 16. Springer Verlag, Heidelberg, pp 323–328
Burnstock G, Cocks T, Crowe R, Kasakov L (1978) Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol 63:125–138
Burnstock G, Cocks T, Kasakov L, Wong HK (1978) Direct evidence for ATP release from non-adrenergic, non-cholinergic ("purinergic") nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol 49:145–149
Burnstock G, Cocks T, Paddle B, Staszewska-Barczak J (1975) Evidence that prostaglandin is responsible for the 'rebound contraction' following stimulation of non-adrenergic, non-cholinergic ('purinergic') inhibitory nerves. Eur J Pharmacol 31:360–362
Burnstock G, Cusack NJ, Hills JM, Mackenzie I, Meghji P (1983) Studies on the stereoselectivity of the P2-purinoceptor. Br J Pharmacol 79:907–913
Burnstock G, Cusack NJ, Meldrum LA (1984) Effects of phosphorothioate analogues of ATP, ADP and AMP on guinea-pig taenia coli and urinary bladder. Br J Pharmacol 82:369–374
Burnstock G, Dumsday B, Smythe A (1972) Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol 44:451–461
Burnstock G, Fischer B, Hoyle CHV, Maillard M, Ziganshin AU, Brizzolara AL, von Isakovics A, Boyer JL, Harden TK, Jacobson KA (1994) Structure activity relationships for derivatives of adenosine 5′-triphosphate as agonists at P2 purinoceptors: heterogeneity within P2X and P2Y subtypes. Drug Dev Res 31:206–219
Burnstock G, Holman ME (1960) Autonomic nerve-smooth muscle transmission. Nature 187:951–952
Burnstock G, Holman ME (1961) The transmission of excitation from autonomic nerve to smooth muscle. J Physiol 155:115–133
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304
Burnstock G, Lavin S (2002) Interstitial cells of Cajal and purinergic signalling. Auton Neurosci 97:68–72
Bushfield M, Kenny BA, Parker N (1996) Facilitation by 5-HT of ATP-mediated electrically-stimulated contractions in the pig urinary bladder. Br J Pharmacol 117, 201P
Callahan SM, Creed KE (1981) Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol 74:353–358
Calvert RC, Banks FC, Thompson CS, Mikhailidis DP, Morgan RJ (2002) Chronic ethanol consumption impairs adrenoceptor- and purinoceptor-mediated relaxations in isolated rat detrusor smooth muscle. BJU Int 89:793–794
Calvert RC, Thompson CS, Burnstock G (2008) ATP release from the human ureter on distension and P2X3 receptor expression on suburothelial sensory nerves. Purinergic Signalling 4:377–381
Canda AE, Cross RL, Chapple CR (2006) Pharmacology of the lower urinary tract and management of overactive bladder. J Turkish German Gynecol Assoc 7:146–158
Canda AE, Turna B, Cinar GM, Nazli O (2007) Physiology and pharmacology of the human ureter: basis for current and future treatments. Urol Int 78:289–298
Carley ME, Cliby WA, Spelsberg TC (2002) P2X3 receptor subunit messenger RNA expression in the female mouse bladder after oophorectomy with or without estrogen replacement. Am J Obstet Gynecol 187:103–106
Carpenter FG, Rubin RM (1967) The motor innervation of the rat urinary bladder. J Physiol 192:609–617
Cervero F, Sann H (1989) Mechanically evoked responses of afferent fibres innervating the guinea-pig's ureter: an in vitro study. J Physiol 412:245–266
Chancellor MB, de Groat WC (1999) Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol 162:3–11
Chancellor MB, Fowler CJ, Apostolidis A, de Groat WC, Smith CP, Somogyi GT, Aoki KR (2008) Drug insight: biological effects of Botulinum toxin a in the lower urinary tract. Nat Clin Pract Urol 5:319–328
Chancellor MB, Kaplan SA, Blaivas JG (1992) The cholinergic and purinergic components of detrusor contractility in a whole rabbit bladder model. J Urol 148:906–909
Chancellor MB, Shenot PJ, Rivas DA, Mandel S, Schwartzman RJ (1996) Urological symptomatology in patients with reflex sympathetic dystrophy. J Urol 155:634–637
Charrua A, Cruz CD, Cruz F, Avelino A (2007) Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol 177:1537–1541
Charrua A, Cruz CD, Narayanan S, Gharat L, Gullapalli S, Cruz F, Avelino A (2009) GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol 181:379–386
Chaudhry A, Downie JW, White TD (1984) Tetrodotoxin-resistant release of ATP from superfused rabbit detrusor muscle during electrical field stimulation in the presence of luciferin– luciferase. Can J Physiol Pharmacol 62:153–156
Chen H-I, Brading AF (1991) The mechanism of action of putative non-adrenergic, non-cholinergic transmitters on the rabbit urinary bladder. J Auton Nerv Syst 33:178–179
Chen X, Gebhart GF (2010) Differential purinergic signaling in bladder sensory neurons of naïve and bladder-inflamed mice. Pain 148:462–472
Cheng Y, Allen W, Walsh C, Mansfield KJ, Burcher E, Moore KH (2009) ATP release during cystometry in women with detrusor overactivity and painful bladder syndrome: contribution to 'urgency'. Neurourol Urodyn 28:838–839
Cheng Y, Mansfield KJ, Allen W, Millard RJ, Burcher E, Moore KH (2013) Correlation between cystometric volumes, ATP release, and pH in women with overactive bladder versus controls. Neurourol Urodyn 32:969–973
Cheng Y, Mansfield K, Burcher E, Moore K (2012) ATP release during bladder filling in women with bladder oversensitivity. J Urol 187:e934
Cheng Y, Mansfield KJ, Sandow SL, Sadananda P, Burcher E, Moore KH (2011) Porcine bladder urothelial, myofibroblast, and detrusor muscle cells: characterization and ATP release. Front Pharmacol 2:27
Cheng S, Scigalla FP, Speroni di Fenizio P, Zhang ZG, Stolzenburg JU, Neuhaus J (2011) ATP enhances spontaneous calcium activity in cultured suburothelial myofibroblasts of the human bladder. PLoS One 6:e25769
Cheng Y, Walsh C, Mansfield K, Allen W, Moore K (2011) Relationship between bacteriuria and ATP concentration in voided urodynamic fluid: a 2-year prospective study. Neurourol Urodyn 30:921
Chesher GB, Thorp RH (1965) The atropine-resistance of the response to intrinsic nerve stimulation of the guinea-pig bladder. Br J Pharmacol 25:288–294
Chess-Williams R, Kang S, McDermott C (2012) Is the release of urothelial ATP, acetylcholine and prostoglandin E2 affected by the chemotherapeutic agent doxorubicin? Neurourol Urodyn 31:1020
Chess-Williams R, Mills K, McDermott C (2012) Acrolein, a metabolite of cyclophosphamide enhances basal ATP release and reactive oxygen species formation in cultured human urothelial cells. Neurourol Urodyn 31:1019
Choo LK (1981) The effect of reactive blue, an antagonist of ATP, on the isolated urinary bladders of guinea-pig and rat. J Pharm Pharmacol 33:248–250
Choo LK, Mitchelson F (1980) The effect of indomethacin and adenosine 5′-triphosphate on the excitatory innervation of the rate urinary bladder. Can J Physiol Pharmacol 58:1042–1048
Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA (2005) Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol 562:859–871
Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA (2008) Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol 294:F821–F829
Chua WC, Liu L, Mansfield KJ, Vaux KJ, Moore KH, Millard RJ, Burcher E (2007) Age-related changes of P2X1 receptor mRNA in the bladder detrusor from men with and without bladder outlet obstruction. Exp Gerontol 42:686–692
Chung SD, Chien CT, Yu HJ (2013) Alterations in peripheral purinergic and muscarinic signaling of rat bladder after long-term fructose-induced metabolic syndrome. Eur J Nutr 52:347–359
Clemow DB, Steers WD, McCarty R, Tuttle JB (1998) Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol 275:R1279–R1286
Cockayne DA, Dunn PM, Zhong Y, Hamilton SG, Cain GR, Knight G, Ruan H-Z, Ping Y, Nunn P, Bei M, McMahon SB, Burnstock G, Ford APDW (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567:621–639
Cockayne DA, Hamilton SG, Zhu Q-M, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–1015
Coelho A, Dinis P, Pinto R, Gorgal T, Silva C, Silva A, Silva J, Cruz CD, Cruz F, Avelino A (2010) Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol 57:884–890
Conte B, Maggi CA, Meli A (1989) Vesico-inhibitory responses and capsaicin-sensitive afferents in rats. Naunyn Schmiedebergs Arch Pharmacol 339:178–183
Corsi M, Pietra C, Toson G, Trist D, Tuccitto G, Artibani W (1991) Pharmacological analysis of 5-hydroxytryptamine effects on electrically stimulated human isolated urinary bladder. Br J Pharmacol 104:719–725
Cotton KD, Hollywood MA, Thornbury KD, McHale NG (1996) Effect of purinergic blockers on outward current in isolated smooth muscle cells of the sheep bladder. Am J Physiol 270:C969–C973
Cowan WD, Daniel EE (1983) Human female bladder and its noncholinergic contractile function. Can J Physiol Pharmacol 61:1236–1246
Crack BE, Pollard CE, Beukers MW, Roberts SM, Hunt SF, Ingall AH, McKechnie KC, IJzerman AP, Leff P (1995) Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharmacol 114:475–481
Creed KE (1979) The role of the hypogastric nerve in bladder and urethral activity of the dog. Br J Pharmacol 65:367–375
Creed KE, Callahan SM, Ito Y (1994) Excitatory neurotransmission in the mammalian bladder and the effects of suramin. Br J Urol 74:736–743
Creed KE, Ishikawa S, Ito Y (1983) Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338:149–164
Creed KE, Ito Y, Katsuyama H (1991) Neurotransmission in the urinary bladder of rabbits and guinea pigs. Am J Physiol 261:C271–C277
Creed KE, Tulloch AG (1978) The effect of pelvic nerve stimulation and some drugs on the urethra and bladder of the dog. Br J Urol 50:398–405
Cristofaro V, Chaudhury A, Goyal RK, Sullivan MP (2013) Impaired purinergic neurotransmission in myosin-Va deficient mouse bladders. Neurourol Urodyn 21:137
Cristofaro V, Yalla SV, Sullivan MP (2012) Altered caveolar mediated purinergic signaling in spontaneously hypertensive rats with detrusor overactivity. J Urol 188:1017–1026
Crowe R, Burnstock G (1985) Perinatal development of adrenergic, cholinergic and non-adrenergic, non-cholinergic nerves and SIF cells in the rabbit urinary bladder. Int J Dev Neurosci 3:89–101
Crowe R, Burnstock G (1989) A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra. J Urol 141:414–422
Crowe R, Burnstock G, Light JK (1988) Intramural ganglia in the human urethra. J Urol 140:183–187
Crowe R, Haven AJ, Burnstock G (1986) Intramural neurons of the guinea-pig urinary bladder: histochemical localization of putative neurotransmitters in cultures and newborn animals. J Auton Nerv Syst 15:319–339
Crowe R, Light JK, Chilton CP, Burnstock G (1985) Vasoactive intestinal polypeptide (VIP)-immunoreactive nerve fibres associated with the striated muscle of the human external urethral sphincter [letter]. Lancet 325:47–48
Cruz F (2013) Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn doi:10.1002/nau.22445
Cusack NJ, Hourani SMO (1984) Some pharmacological and biochemical interactions of the enantiomers of adenylyl 5′-(β, γ-methylene)-diphosphonate with the guinea-pig urinary bladder. Br J Pharmacol 82:155–159
Cusack NJ, Hourani SMO, Loizou GD, Welford LA (1987) Pharmacological effects of isopolar phosphonate analogues of ATP on P2-purinoceptors in guinea-pig taenia coli and urinary bladder. Br J Pharmacol 90:791–795
Cusack NJ, Hourani SMO, Welford LA (1988) The role of ectonucleotidases in pharmacological responses to nucleotide analogues. In: Paton DM (ed) Adenosine and adenine nucleotides. Taylor & Francis, London, pp 93–100
Dagnino-Acosto A, Munoz A, Smith CP, Boone T, Somogyi G (2011) Elevated extracellular Ca2+ levels affect the purinergic response in rat bladder smooth muscle. Neurourol Urodyn 30:226–227
D'Agostino G, Condino AM, Calvi V, Boschi F, Gioglio L, Barbieri A (2012) Purinergic P2X3 heteroreceptors enhance parasympathetic motor drive in isolated porcine detrusor, a reliable model for development of P2X selective blockers for detrusor hyperactivity. Pharmacol Res 65:129–136
Dahlén SE, Hedqvist P (1980) ATP, β-γ-methylene-ATP, and adenosine inhibit non-cholinergic non-adrenergic transmission in rat urinary bladder. Acta Physiol Scand 109:137–142
Dail WG, Evan AP, Gerritsen GC, Dulin WE (1977) Abnormalities in pelvic visceral nerves. A basis for neurogenic bladder in the diabetic Chinese hamster. Invest Urol 15:161–166
Daly DM, Collins VM, Chapple CR, Grundy D (2011) The afferent system and its role in lower urinary tract dysfunction. Curr Opin Urol 21:268–274
Daly DM, Collins VM, McKay NG, Sellers DJ, Chapple CR, Grundy D (2012) Botulinum neurotoxin A (BoNT/A) attenuates bladder afferent nerve firing and prevents ATP release from the urothelium. Curr Mol Med 12:e374
Daly D, Nocchi L, Schluter M, McKay N, Keating C, Chapple C, Grundy D (2012) Aged related changes in purinergic signalling, afferent firing and receptor expression in the mouse bladder. Neurourol Urodyn 31:955–833
Daneshgari F, Liu G, Imrey PB (2006) Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol 176:380–386
Dang K, Bielefeldt K, Gebhart GF (2005) Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25:3973–3984
Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF (2008) Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99:49–59
Dasgupta J, Elliott RA, Doshani A, Tincello DG (2006) Enhancement of rat bladder contraction by artificial sweeteners via increased extracellular Ca2+ influx. Toxicol Appl Pharmacol 217:216–224
Dasgupta J, Tincello DG (2009) Interstitial cystitis/bladder pain syndrome: an update. Maturitas 64:212–217
Davidson RA, McCloskey KD (2005) Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol 173:1385–1390
de Groat WC (1975) Nervous control of the urinary bladder of the cat. Brain Res 87:201–211
de Groat WC (1986) Spinal cord projections and neuropeptides in visceral afferent neurons. In: Cervero F, Morrison JFB (eds) Visceral sensation. Progress in brain reseach, vol 67. Elservier, Amsterdam, pp 165–188
de Groat WC (1987) Neuropeptides in pelvic afferent pathways. Experientia 43:801–813
de Groat WC (2006) Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147:S25–S40
de Groat WC, Booth AM (1980) Inhibition and facilitation in parasympathetic ganglia of the urinary bladder. Fed Proc 39:2990–2996
de Groat WC, Booth AM (1993) Synaptic transmission in pelvic ganglia. In: Maggi C (ed) The autonomic nervous system, vol. 3. Nervous control of the urogenital system. Harwood Academic Publishers, Chur, pp 291–348
de Groat WC, Booth AM, Krier J, Milne RJ, Morgan C, Nadelhaft I (1979) Neural control of the urinary bladder and large intestine. In: Brooks CM, Koizumi K, Sato A (eds) Integrative functions of the autonomic nervous system. Elsevier/North Holland Biomedical Press, Amaterdam, pp 50–67
de Groat WC, Booth WC, Yoshimura M (1993) Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA (ed) The autonomic nervous system. Volume 3. Nervous control of the urogenital system. Harwood Academic Publishers, Chur, pp 227–290
de Groat WC, Kawatani M (1989) Enkephalinergic inhibition in parasympathetic ganglia of the urinary bladder of the cat. J Physiol 413:13–29
de Groat WC, Kawatani M (1989) Reorganisation of sympathetic preganglionic connections in cat bladder ganglia following parasympathetic denervation. J Physiol 409:431–449
de Groat WC, Kawatani M, Hisamitsu T, Cheng C-L, Ma C-P, Thor K, Steers W, Roppolo JR (1990) Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst 30:S71–S77
de Groat WC, Saum WR (1972) Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol 220:297–314
de Groat WC, Saum WR (1976) Synaptic transmission in parasympathetic ganglia in the urinary bladder of the cat. J Physiol 256:137–158
de Groat WC, Theobald RJ (1976) Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol 259:223–237
De Mey J, Burnstock G, Vanhoutte PM (1979) Modulation of the evoked release of noradrenaline in canine saphenous vein via presynaptic receptors for adenosine but not ATP. Eur J Pharmacol 55:401–405
De Sy W, Lacroix E, Leusen I (1974) An analysis of the urinary bladder response to hypogastric nerve stimulation in the cat. Invest Urol 11:508–516
De Wachter S (2011) Afferent signaling from the bladder: species differences evident from extracellular recordings of pelvic and hypogastric nerves. Neurourol Urodyn 30:647–652
Dean DM, Downie JW (1978) Contribution of adrenergic and 'purinergic' neurotransmission to contraction in rabbit detrusor. J Pharmacol Exp Ther 207:431–445
Dean DM, Downie JW (1978) Interaction of prostaglandins and adenosine 5′-triphosphate in the noncholinergic neurotransmission in rabbit detrusor. Prostaglandins 16:245–251
Dhulipala PD, Wang YX, Kotlikoff MI (1998) The human P2X4 receptor gene is alternatively spliced. Gene 207:259–266
Dokita S, Morgan WR, Wheeler MA, Yoshida M, Latifpour J, Weiss RM (1991) N G-Nitro-l-arginine inhibits non-adrenergic, non-cholinergic relaxation in rabbit urethral smooth muscle. Life Sci 48:2429–2436
Donoso MV, Salas C, Sepulveda G, Lewin J, Fournier A, Huidobro-Toro JP (1994) Involvement of ETA receptors in the facilitation by endothelin-1 of non-adrenergic non-cholinergic transmission in the rat urinary bladder. Br J Pharmacol 111:473–482
Downie JW, Larsson C (1981) Prostaglandin involvement in contractions evoked in rabbit detrusor by field stimulation and by adenosine 5′-triphosphate. Can J Physiol Pharmacol 59:253–260
Dunn PM, Blakeley AGH (1988) Suramin: a reversible P 2-purinceptor antagonist in the mouse vas deferens. Br J Pharmacol 93:243–245
Dunn M, Smith JC, Ardran GM (1974) Prolonged bladder distension as a treatment of urgency and urge incontinence of urine. Br J Urol 46:645–652
Dunning-Davies BM, Fry CH, Mansour D, Ferguson DR (2013) The regulation of ATP release from the urothelium by adenosine and transepithelial potential. BJU Int 111:505–513
Dutton JL, Hansen MA, Balcar VJ, Barden JA, Bennett MR (1999) Development of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol 28:4–16
Ehlert FJ (2003) Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci 74:355–366
Ehlert FJ, Ahn S, Pak KJ, Park GJ, Sangnil MS, Tran JA, Matsui M (2007) Neuronally released acetylcholine acts on the M2 muscarinic receptor to oppose the relaxant effect of isoproterenol on cholinergic contractions in mouse urinary bladder. J Pharmacol Exp Ther 322:631–637
Eika B, Salling LN, Loft L, Laurberg S, Lundbeck F (1988) Effect of estrogen on NANC transmission in bladder of mature female rats. Neurourol Urodyn 7:201–203
Ekman M, Andersson KE, Arner A (2006) Developmental regulation of nerve and receptor mediated contractions of mammalian urinary bladder smooth muscle. Eur J Pharmacol 532:99–106
Ekström J, Elmér M (1980) Compensatory increase of responses to nerve stimulation of the partially denervated rat urinary bladder. Acta Physiol Scand 110:21–29
Ekström J, Henningsson AC, Henningsson S, Malmberg L (1984) Hyperplasia and hypertrophia in the denervated and distended rat urinary bladder. Acta Physiol Scand 122:45–48
Ekström J, Malmberg L (1984) Development of supersensitivity to methacholine in the rat detrusor following either parasympathetic denervation or decentralization. Acta Physiol Scand 122:175–179
Ekström J, Uvelius B (1981) Length–tension relations of smooth muscle from normal and denervated rat urinary bladders. Acta Physiol Scand 112:443–447
Ellenberg M (1980) Development of urinary bladder dysfunction in diabetes mellitus. Ann Intern Med 92:321–323
El-Mas MM, Elmallah AI, Omar AG, Sharabi F (1999) Dopamine modulates peripheral purinergic neurotransmission through multiple presynaptic receptors: tissue-dependent effects. Pharmacol Res 39:11–19
Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR (2001) Distribution of P2X1 and P2X3 receptors in the rat and human urinary bladder. Pharmacology 63:120–128
Erickson K, Buffington CA, Kanai AJ, de Groat WC, Bullo A, D'Alatri L, Edwards D, Birder LA (1990) Alterations in nitric oxide (NO) production of NK2 immunoreactivity in urinary bladder from cats with feline interstitial cystitis. Soc Neurosci Abstr 24:1619
Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A (1995) Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol Pharmacol 48:178–183
Fabiyi AC, Brading AF (2006) The use of the isolated mouse whole bladder for investigating bladder overactivity. J Pharmacol Exp Ther 319:1386–1394
Faerman I, Glocer L, Celener D, Jadzinsky M, Fox D, Maler M, Alvarez E (1973) Autonomic nervous system and diabetes. Histological and histochemical study of the autonomic nerve fibers of the urinary bladder in diabetic patients. Diabetes 22:225–237
Ferguson D, Christopher N (1996) Urinary bladder function and drug development. Trends Pharmacol Sci 17:161–165
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505:503–511
Flood HD, Downie JW, Awad SA (1988) Influence of filling rates and sympathectomy on bladder compliance in the chloralose-anaesthetised cat. Neurourol Urodyn 7:377–384
Ford AP, Cockayne DA (2011) ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol 202:485–526
Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA (2006) Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147:S132–S143
Fowler CJ (2006) Integrated control of lower urinary tract — clinical perspective. Br J Pharmacol 147:S14–S24
Fowler CJ, Beck RO, Gerrard S, Betts CD, Fowler CG (1994) Intravesical capsaicin for treatment of detrusor hyperreflexia. J Neurol Neurosurg Psychiatry 57:169–173
Fowler CJ, Griffiths D, de Groat WC (2008) The neural control of micturition. Nat Rev Neurosci 9:453–466
Fowler CJ, Jewkes D, McDonald WI, Lynn B, de Groat WC (1992) Intravesical capsaicin for neurogenic bladder dysfunction. Lancet 339:1239
Friedland GW, Perkash I (1983) Neuromuscular dysfunction of the bladder and urethra. Semin Roentgenol 18:255–266
Frimodt-Moller C (1980) Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med 92:318–321
Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP (2004) Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology 63:24–31
Fry CH, Meng E, Young JS (2010) The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154:3–13
Fry CH, Wu C (2000) Determinants of mechanical activity in detrusor smooth muscle. J Physiol 523:61P
Fry CH, Young JS, Jabr RI, McCarthy C, Ikeda Y, Kanai AJ (2012) Modulation of spontaneous activity in the overactive bladder: the role of P2Y agonists. Am J Physiol Renal Physiol 302:F1447–F1454
Fuder H, Muscholl E (1995) Heteroreceptor-mediated modulation of noradrenaline and acetylcholine release from peripheral nerves. Rev Physiol Biochem Pharmacol 126:265–412
Fuder H, Muth U (1993) ATP and endogenous agonists inhibit evoked [3H]-noradrenaline release in rat iris via A1 and P2y-like purinoceptors. Naunyn Schmiedebergs Arch Pharmacol 348:352–357
Fujii K (1987) Electrophysiological evidence that adenosine triphosphate (ATP) is a cotransmitter with acetylcholine (ACh) in isolated guinea-pig, rabbit and pig urinary bladder. Proc Physiol Soc 394:26P
Fujii K (1988) Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol 404:39–52
Fujii K, Foster CD, Brading AF, Parekh AB (1990) Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol 99:779–785
Fujii R, Oshima N (1986) Control of chromatophore movements in teleost fish. Zool Sci 3:13–47
Galloway NT, Gabale DR, Irwin PP (1991) Interstitial cystitis or reflex sympathetic dystrophy of the bladder? Semin Urol 9:148–153
Garcia-Pascual A, Costa G, Garcia-Sacristan A, Andersson K-E (1991) Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand 141:531–539
Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117:3453–3462
Gever J, Cockayne DA, Dillon MP, Burnstock G, Ford APDW (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537
Ghoneim MA, Fretin JA, Gagnon DJ, Lebel E, Van Lier J, Arsenault A, Susset JG (1976) The influence of vesical distension on the urethral resistance to flow: a possible role for prostaglandins? J Urol 116:739–743
Ghoniem GM, Shoukry MS (1991) Atropine resistance phenomenon in human bladders of myelodysplastic children. Neurourol Urodyn 10:304
Giglio D, Aronsson P, Eriksson L, Tobin G (2007) In vitro characterization of parasympathetic and sympathetic responses in cyclophosphamide-induced cystitis in the rat. Basic Clin Pharmacol Toxicol 100:96–108
Giglio D, Delbro DS, Tobin G (2001) On the functional role of muscarinic M2 receptors in cholinergic and purinergic responses in the rat urinary bladder. Eur J Pharmacol 428:357–364
Gill HS, Wein AJ, Ruggieri MR, Whitmore KE, Levin RM (1989) Functional and biochemical alterations in the rabbit urinary bladder following ileocystoplasty. J Urol 142:860–864
Gilpin CJ, Dixon JS, Gilpin SA, Gosling JA (1983) The fine structure of autonomic neurons in the wall of the human urinary bladder. J Anat 137:705–713
Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA (2008) PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36:310–320
Göcmen C, Giesselman B, de Groat WC (2005) Effect of neocuproine, a copper(i) chelator, on rat bladder function. J Pharmacol Exp Ther 312:1138–1143
Gómez-Pinilla PJ, Pozo MJ, Camello PJ (2007) Aging impairs neurogenic contraction in guinea pig urinary bladder: role of oxidative stress and melatonin. Am J Physiol Regul Integr Comp Physiol 293:R793–R803
Gómez-Pinilla PJ, Pozo MJ, Camello PJ (2011) Aging differentially modifies agonist-evoked mouse detrusor contraction and calcium signals. Age (Dordr) 33:81–88
Gonçalves RG, Gabrich L, Rosário A Jr, Takiya CM, Ferreira ML, Chiarini LB, Persechini PM, Coutinho-Silva R, Leite M Jr (2006) The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70:1599–1606
Gowing NFC (1960) Pathological changes in the bladder following irradiation. Br J Radiol 33:484–487
Grandadam F, Lluel P, Palea S, Martin DJ (1999) Pharmacological and urodynamic changes in rat urinary bladder function after multiple pregnancies. BJU Int 84:861–866
Grundy L, Chess-Williams R, Grundy D (2012) Primary mouse urothelial cell response to ATP is mediated by P2X but not TRPV1 receptors. Neurourol Urodyn 31:1021
Gu J, Polak JM, Deane A, Cocchia D, Michetti F (1984) Increase of S-100 immunoreactivity in the urinary bladder from patients with multiple sclerosis, an indication of peripheral neuronal lesion. Am J Clin Pathol 82:649–654
Gür S, Karahan ST (1997) Effects of adenosine 5′-triphosphate, adenosine and acetylcholine in urinary bladder and colon muscles from streptozotocin diabetic rats. Arzneimittelforschung 47:1226–1229
Hammer K, Sann H, Pierau F-K (1993) Functional properties of mechanosensitive units from the chicken ureter in vitro. Pflugers Arch 425:353–361
Hansen MA, Balcar VJ, Barden JA, Bennett MR (1998) The distribution of single P2X1-receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol 27:529–539
Harrison SC, Ferguson DR, Doyle PT (1990) Effect of bladder outflow obstruction on the innervation of the rabbit urinary bladder. Br J Urol 66:372–379
Harrison SC, Ferguson DR, Hanley MR (1990) Effect of capsaicin on the rabbit urinary bladder. What is the function of sensory nerves that contain substance P? Br J Urol 66:155–161
Harvey RA, Skennerton DE, Newgreen D, Fry CH (2002) The contractile potency of adenosine triphosphate and ecto-adenosine triphosphatase activity in guinea pig detrusor and detrusor from patients with a stable, unstable or obstructed bladder. J Urol 168:1235–1239
Hashimoto S, Kigoshi S, Muramatsu I (1992) Neurogenic responses of urethra isolated from the dog. Eur J Pharmacol 213:117–123
Hashimoto S, Kigoshi S, Muramatsu I (1993) Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur J Pharmacol 231:209–214
Hashimoto M, Kokubun S (1995) Contribution of P2-purinoceptors to neurogenic contraction of rat urinary bladder smooth muscle. Br J Pharmacol 115:636–640
Hashitani H, Bramich NJ, Hirst GD (2000) Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524(Pt 2):565–579
Hashitani H, Edwards FR (1999) Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol 514:459–470
Hashitani H, Suzuki H (1995) Electrical and mechanical responses produced by nerve stimulation in detrusor smooth muscle of the guinea-pig. Eur J Pharmacol 284:177–183
Hawranko AA, Barrick S, Birder S, de Groat WC (1999) Effects of capsaicin and cyclophosphamide on the distension-dependent release of ATP into the lumen of the lower urinary tract and peripheral administration of purinergic agonists on the micturition reflex in the rat. Soc Neurosci Abst 25:1171
Hegde SS, Mandel DA, Wilford MR, Briaud S, Ford APDW, Eglen RM (1998) Evidence for purinergic neurotransmission in the urinary bladder of pithed rats. Eur J Pharmacol 349:75–82
Henderson VE (1923) The action of atropine on intestine and urinary bladder. Arch Int Pharmacodyn Ther 27:205–211
Henning RH (1997) Purinoceptors in neuromuscular transmission. Pharmacol Ther 74:115–128
Heppner TJ, Bonev AD, Nelson MT (2005) Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564:201–212
Hernández M, Barahona MV, Bustamante S, García-Sacristín A, Orensanz LM (1999) A2B adenosine receptors mediate relaxation of the pig intravesical ureter: adenosine modulation of non adrenergic non cholinergic excitatory neurotransmission. Br J Pharmacol 126:969–978
Hernández M, Malone-Lee J, Knight GE, Wildman S, Burnstock G (2009) Role of ATP and related purines in the inhibitory neurotransmission of the pig urinary bladder neck. Br J Pharmacol 157:1463–1473
Hills J, Meldrum LA, Klarskov P, Burnstock G (1984) A novel non-adrenergic, non-cholinergic nerve-mediated relaxation of the pig bladder neck: an examination of possible neurotransmitter candidates. Eur J Pharmacol 99:287–293
Hindmarsh JR, Idowu OA, Yeates WK, Zar MA (1977) Pharmacology of electrically evoked contractions of human bladder. Br J Pharmacol 61:115P
Hisayama T, Shinkai M, Takayanagi I, Toyoda T (1988) Mechanism of action of nicotine in isolated urinary bladder of guinea-pig. Br J Pharmacol 95:465–472
Hogaboom GK, O'Donnell JP, Fedan JS (1980) Purinergic receptors: photoaffinity analog of adenosine triphosphate is a specific adenosine triphosphate antagonist. Science 208:1273–1275
Holck MI, Marks BH (1978) Purine nucleoside and nucleotide interactions on normal and subsensitive alpha adrenoreceptor responsiveness in guinea-pig vas deferens. J Pharmacol Exp Ther 205:104–117
Holm-Bentzen M, Lose G (1987) Pathology and pathogenesis of interstitial cystitis. Urology 29:8–13
Holt SE, Cooper M, Wyllie JH (1985) Evidence for purinergic transmission in mouse bladder and for modulation of responses to electrical stimulation by 5-hydroxytryptamine. Eur J Pharmacol 116:105–111
Holzer P (1991) Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43:143–201
Hotta H, Masunaga K, Miyazaki S, Watanabe N, Kasuya Y (2012) A gentle mechanical skin stimulation technique for inhibition of micturition contractions of the urinary bladder. Auton Neurosci 167:12–20
Hourani SMO (1999) Postnatal development of purinoceptors in rat visceral smooth muscle preparations. Gen Pharmacol 32:3–7
Hourani SMO, Chown JA (1989) The effects of some possible inhibitors of ectonucleotidases on the breakdown and pharmacological effects of ATP in the guinea-pig urinary bladder. Gen Pharmacol 20:413–416
Hourani SMO, Welford LA, Cusack NJ (1985) l-AMP-PCP, an ATP receptor agonist in guinea-pig bladder, is inactive on taenia coli. Eur J Pharmacol 108:197–200
Hoyes AD (1984) Fine structureand response to capsaicin of primary afferent nociceptive axons in the rat and guinea-pig ureter. In: Hamann W, Iggo A (eds) Sensory receptor mechanisms. World Scientific Publ, Singapore, pp 25–34
Hoyes AD, Barber P, Martin BG (1975) Comparative ultrastructure of the nerves innervating the muscle of the body of the bladder. Cell Tissue Res 164:133–144
Hoyle CH (1994) Non-adrenergic, non-cholinergic control of the urinary bladder. World J Urol 12:233–244
Hoyle CHV, Burnstock G (1985) Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α, β-methylene ATP. Eur J Pharmacol 114:239–240
Hoyle CHV, Burnstock G (1993) Postganglionic efferent transmission to the bladder and urethra. In: Maggi C (ed) The autonomic nervous system, vol. 3. Nervous control of the urogenital system. Harwood Academic Publishers, Switzerland, pp 349–383
Hoyle CH, Chakrabarti G, Pendleton NP, Andrews PL (1998) Neuromuscular transmission and innervation in the urinary bladder of the insectivore Suncus murinus. J Auton Nerv Syst 69:31–38
Hoyle CHV, Chapple C, Burnstock G (1989) Isolated human bladder: evidence for an adenine dinucleotide acting on P2X-purinoceptors and for purinergic transmission. Eur J Pharmacol 174:115–118
Hoyle CHV, Knight GE, Burnstock G (1990) Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol 99:617–621
Hoyle CHV, Lincoln J, Burnstock G (1994) Neural control of pelvic organs. In: Rushton DN (ed) Handbook of neuro-urology. Marcel Dekker, New York, pp 1–54
Hoyle CHV, Ralevic V, Lincoln J, Knight GE, Goss-Sampson MA, Milla PJ, Burnstock G (1995) Effects of vitamin E deficiency on autonomic neuroeffector mechanisms in the rat caecum, vas deferens and urinary bladder. J Physiol 487:773–786
Hu ST, Gever J, Nunn PA, Ford AP, Zhu Q-M (2004) Cystometric studies with ATP, PPADS and TNP-ATP in conscious and anaesthetised C57BL/6 mice. J Urol 171:461–462
Huddart H, Butler DJ (1986) Field stimulation responses of rat urinary bladder detrusor smooth-muscle. Dependence upon slow calcium channel activity determined by K+ depolarization and calcium antagonists. Gen Pharmacol 17:695–703
Hudman D, Elliott RA, Norman RI (2000) Inhibition of the contractile response of the rat detrusor muscle by the β2-adrenoceptor agonist clenbuterol. Eur J Pharmacol 392:79–85
Husted S, Andersson K-E, Sommer L, Østergaard JR (1980) Anticholinergic and calcium antagonistic effects of terodiline in rabbit urinary bladder. Acta Pharmacol Toxicol 46(Suppl 1):20–30
Husted S, Sjögren C, Andersson K-E (1980) Mechanisms of the responses to non-cholinergic, non-adrenergic nerve stimulation and to ATP in isolated rabbit urinary bladder: evidence for ADP evoked prostaglandin release. Acta Pharmacol Toxicol 47:84–92
Husted S, Sjögren C, Andersson K-E (1980) Role of prostaglandins in the responses of rabbit detrusor to non-cholinergic, non-adrenergic nerve stimulation and to ATP. Arch Int Pharmacodyn Ther 246:84–97
Husted S, Sjögren C, Andersson K-E (1983) Direct effects of adenosine and adenine nucleotides on isolated human urinary bladder and their influence on electrically induced contractions. J Urol 130:392–398
Hutton KA, Trejdosiewicz LK, Thomas DF, Southgate J (1993) Urothelial tissue culture for bladder reconstruction: an experimental study. J Urol 150:721–725
Iacovou JW, Hill SJ, Birmingham AT (1990) Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J Urol 144:775–779
Igawa Y, Mattiasson A, Andersson K-E (1993) Functional importance of cholinergic and purinergic neurotransmission for micturition contraction in the normal, unanaesthetized rat. Br J Pharmacol 109:473–479
Igawa Y, Mattiasson A, Andersson K-E (1994) Micturition and premicturition contractions in unanesthetized rats with bladder outlet obstruction. J Urol 151:244–249
Igawa Y, Zhang X, Nishizawa O, Umeda M, Iwata A, Taketo MM, Manabe T, Matsui M, Andersson KE (2004) Cystometric findings in mice lacking muscarinic M2 or M3 receptors. J Urol 172:2460–2464
Ikeda Y, Zabbarova IV, Birder LA, de Groat WC, McCarthy CJ, Hanna-Mitchell AT, Kanai AJ (2012) Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol 62:1157–1164
Imagawa J, Akima M, Sakai K (1989) Functional evaluation of sympathetically mediated responses in in vivo lower urinary tract of dogs. J Pharmacol Methods 22:103–111
Imamura T, Ishizuka O, Aizawa N, Zhong C, Ogawa T, Nakayama T, Tanabe T, Nishizawa O (2008) Gosha-jinki-gan reduces transmitter proteins and sensory receptors associated with C fiber activation induced by acetic acid in rat urinary bladder. Neurourol Urodyn 27:832–837
Inoue R, Brading AF (1990) The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol 100:619–625
Inoue R, Brading AF (1991) Human, pig and guinea-pig bladder smooth muscle cells generate similar inward currents in response to purinoceptor activation. Br J Pharmacol 103:1840–1841
Iosif CS, Batra S, Ek A, Astedt B (1981) Estrogen receptors in the human female lower uninary tract. Am J Obstet Gynecol 141:817–820
Iravani MM, Luheshi GN, Zar MA (1988) Inhibition of non-cholinergic motor transmission in isolated rat bladder by nifedipine. J Physiol 410:61P
Iravani MM, Zar MA (1994) Neuropeptide Y in rat detrusor and its effect on nerve-mediated and acetylcholine-evoked contractions. Br J Pharmacol 113:95–102
Ishihama H, Momota Y, Yanase H, Wang X, de Groat WC, Kawatani M (2006) Activation of α1D adrenergic receptors in the rat urothelium facilitates the micturition reflex. J Urol 175:358–364
Ito Y, Kimoto Y (1985) The neural and non-neural mechanisms involved in urethral activity in rabbits. J Physiol 367:57–72
James MJ, Birmingham AT, Hill SJ (1993) Partial mediation by nitric oxide of the relaxation of human isolated detrusor strips in response to electrical field stimulation. Br J Clin Pharmacol 35:366–372
Jancsó G, Maggi CA (1987) Distribution of capsaicin-sensitive urinary bladder afferents in the rat spinal cord. Brain Res 418:371–376
Jänig W, Morrison JFB (1986) Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. In: Cervero F, Morrison JFB (eds) Visceral sensation, progress in brain reseach, vol 67. Elservier Science B.V, Amsterdam, pp 87–114
Jenes Á, Ruzsnavszky F, Telek A, Szigeti GP, Csernoch L (2012) A possible role of the cholinergic and purinergic receptor interaction in the regulation of the rat urinary bladder function. J Muscle Res Cell Motil 32:421–431
Ji G, Feldman ME, Deng KY, Greene KS, Wilson J, Lee JC, Johnston RC, Rishniw M, Tallini Y, Zhang J, Wier WG, Blaustein MP, Xin HB, Nakai J, Kotlikoff MI (2004) Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle. J Biol Chem 279:21461–21468
Jiang CH, Lindström S (1999) Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol 517:599–605
Johns A (1981) The effect of indomethacin and substance P on the guinea pig urinary bladder. Life Sci 29:1803–1809
Johns A, Paton DM (1977) Effect of indomethacin on atropine-resistant transmission in rabbit and monkey urinary bladder: evidence for involvement of prostaglandins in transmission. Prostaglandins 13:245–254
Johnson RH, Eisenhofer G, Lambie DG (1986) The effects of acute and chronic ingestion of ethanol on the autonomic nervous system. Drug Alcohol Depend 18:319–328
Jörgensen L, Mortensen SO, Colstrup H, Andersen JT (1985) Bladder distension in the management of detrusor instability. Scand J Urol Nephrol 19:101–104
Juszczak K, Ziomber A, Wyczolkówski M, Thor PJ (2010) Hyperosmolarity alters micturition: a comparison of urinary bladder motor activity in hyperosmolar and cyclophosphamide-induced models of overactive bladder. Can J Physiol Pharmacol 88:899–906
Kaan TK, Yip PK, Grist J, Cefalu JS, Nunn PA, Ford AP, Zhong Y, McMahon SB (2010) Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci 30:4503–4507
Kadima S, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP (2010) Regulation of urethral tone by ATP. Irish J Med Sci 179:S286–S287
Kageyama A, Fujino T, Taki Y, Kato Y, Nozawa Y, Ito Y, Yamada S (2008) Alteration of muscarinic and purinergic receptors in urinary bladder of rats with cyclophosphamide-induced interstitial cystitis. Neurosci Lett 436:81–84
Kageyama S, Fujita K, Suzuki K, Shinbo H, Masuda N, Uchida W (2000) Effect of age on the responses of rat bladder detrusor strips to adenosine triphosphate. BJU Int 85:899–904
Kang SH, Chess-Williams R, Anoopkumar-Dukie S, McDermott C (2013) Induction of inflammatory cytokines and alteration of urothelial ATP, acetylcholine and prostaglandin E2 release by doxorubicin. Eur J Pharmacol 700:102–109
Karsenty G, Elzayat E, Delapparent T, St-Denis B, Lemieux MC, Corcos J (2007) Botulinum toxin type A injections into the trigone to treat idiopathic overactive bladder do not induce vesicoureteral reflux. J Urol 177:1011–1014
Kasakov L, Burnstock G (1983) The use of the slowly degradable analog, α, β-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol 86:291–294
Kasakov LN, Vlaskovska MV (1985) Profile of prostaglandins generated in the detrusor muscle of rat urinary bladder: effects of adenosine triphosphate and adenosine. Eur J Pharmacol 113:431–436
Katsuragi T, Kuratomi L, Furukawa T (1986) Clonidine-evoked selective P1-purinoceptor antagonism of contraction of guinea-pig urinary bladder. Eur J Pharmacol 121:119–122
Katsuragi T, Usune S, Furukawa T (1990) Antagonism by nifedipine of contraction and Ca2+-influx evoked by ATP in guinea-pig urinary bladder. Br J Pharmacol 100:370–374
Keast JR, de Groat WC (1989) Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. J Comp Neurol 288:387–400
Keast JR, Kawatani M, de Groat WC (1990) Sympathetic modulation of cholinergic transmission in cat vesical ganglia is mediated by α1- and α2-adrenoceptors. Am J Physiol 258:R44–R50
Keating MA, Duckett JW, Snyder HM, Wein AJ, Potter L, Levin RM (1990) Ontogeny of bladder function in the rabbit. J Urol 144:766–769
Kelley SP, Birch R, Scott-Ward TS, Peppiatt-Wildman CM, Farmer C, Delaney M, Wildman SS (2013) Urinary ATP and bacteria in shed urothelial cells as a superior diagnostic marker for urinary tract infection in renal transplant recipients. FASEB J 27:646.9
Kerr DIB, Krantis A (1979) A new class of ATP antagonist. Proc Australian Phys Pharmacol Soc 10:156P
Khan MA, Mumtaz FH, Morgan RJ (2000) Does the temperature of lignocaine hydrochloride gel affect instillation discomfort in the male urethra? BJU Int 86:404–405
Khandelwal P, Abraham SN, Apodaca G (2009) Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297:F1477–F1501
Khattab MM, Al-Hrasen MN (2006) Contractile activity of ATP and diadenosine tetraphosphate on urinary bladder in the rats: role of superoxide anion and urothelium. Auton Autacoid Pharmacol 26:149–156
Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP (2004) Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int 45:987–993
Kihara K, de Groat WC (1997) Sympathetic efferent pathways projecting to the bladder neck and proximal urethra in the rat. J Auton Nerv Syst 62:134–142
Kim DY, Hawranko AA, Fraser MO, Yoshiyama M, Chancellor MB, Cheng CL, de Groat WC (1999) The effects of spinal and peripheral administration of purinergic agonists on the micturition reflex in the rat. Soc Neurosci Abstr 25:1170
Kinder RB, Mundy AR (1985) Atropine blockade of nerve-mediated stimulation of the human detrusor. Br J Urol 57:418–421
King JA, Huddart H, Staff WG (1997) Purinergic modulation of rat urinary bladder detrusor smooth muscle. Gen Pharmacol 29:597–604
King BF, Knowles I, Burnstock G, Ramage A (2004) Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetised rats. Br J Pharmacol 142:519–530
Kishii K, Hisayama T, Takayanagi I (1992) Comparison of contractile mechanisms by carbachol and ATP in detrusor strips of rabbit urinary bladder. Jpn J Pharmacol 58:219–229
Kitta T, Chancellor MB, de Groat WC, Kuno S, Nonomura K, Yoshimura N (2012) Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. J Urol 187:1890–1897
Kitta T, Mitsui T, Kanno Y, Moriya K, Nonomura K, Yoshimura N (2013) Interaction between adenosine A2A receptors and dopaminergic receptors in the central nervous system (CNS) control of micturition reflex. J Urol 189:e702
Klarskov P (1987) Non-cholinergic, non-adrenergic nerve-mediated relaxation of pig and human detrusor muscle in vitro. Br J Urol 59:414–419
Klarskov P (1987) Non-cholinergic, non-adrenergic inhibitory nerve responses of bladder outlet smooth muscle in vitro. Br J Urol 60:337–342
Knight GE, Bodin P, de Groat WC, Burnstock G (2002) ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol 282:F281–F288
Knight GE, Brizzolara AL, Soediono P, Karoon P, Burnstock G (1995) Chronic ethanol consumption affects cholinoceptor- and purinoceptor-mediated contractions of the isolated rat bladder. Alcohol 12:183–188
Koley B, Koley J, Saha JK (1984) The effects of nicotine on spontaneous contractions of cat urinary bladder in situ. Br J Pharmacol 83:347–355
Kolta MG, Wallace LJ, Gerald MC (1985) Streptozocin-induced diabetes affects rat urinary bladder response to autonomic agents. Diabetes 34:917–921
Koziol JA, Clark DC, Gittes RF, Tan EM (1993) The natural history of interstitial cystitis: a survey of 374 patients. J Urol 149:465–469
Krell RD, McCoy JL, Ridley PT (1981) Pharmacological characterization of the excitatory innervation to the guinea-pig urinary bladder in vitro: evidence for both cholinergic and non-adrenergic–non-cholinergic neurotransmission. Br J Pharmacol 74:15–22
Kropp BP (1998) Small-intestinal submucosa for bladder augmentation: a review of preclinical studies. World J Urol 16:262–267
Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA (1995) Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology 46:396–400
Kruse MN, Belton AL, de Groat WC (1993) Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol 264:R1157–R1163
Kruse R, Säve S, Persson K (2012) Adenosine triphosphate induced P2Y2 receptor activation induces proinflammatory cytokine release in uroepithelial cells. J Urol 188:2419–2425
Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H, Kohri K (2008) Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn 27:330–340
Kudlacz EM, Chun AL, Skau KA, Gerald MC, Wallace LJ (1988) Diabetes and diuretic-induced alterations in function of rat urinary bladder. Diabetes 37:949–955
Kudlacz EM, Gerald MC, Wallace LJ (1989) Sensory nerves and urinary bladder function: effects of diabetes, capsaicin and acrylamide treatment. Gen Pharmacol 20:31–34
Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA (2008) Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol 294:F971–F981
Kullmann FA, Artim DE, Birder LA, de Groat WC (2008) Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci 28:1977–1987
Kullmann FA, Shah MA, Birder LA, de Groat WC (2009) Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296:F892–F901
Kumar V, Chapple CC, Chess-Williams R (2004) Characteristics of adenosine triphosphate release from porcine and human normal bladder. J Urol 172:744–747
Kumar V, Chapple CR, Rosario D, Tophill PR, Chess-Williams R (2010) In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur Urol 57:1087–1092
Kumar V, Chapple CR, Surprenant AM, Chess-Williams R (2007) Enhanced adenosine triphosphate release from the urothelium of patients with painful bladder syndrome: a possible pathophysiological explanation. J Urol 178:1533–1536
Kumar V, Cross RL, Chess-Williams R, Chapple CR (2005) Recent advances in basic science for overactive bladder. Curr Opin Urol 15:222–226
Kuo DC, Hisamitsu T, de Groat WC (1983) The function of efferent projections from the lumbosacral sympathetic chain to the urinary bladder in the cat. Soc Neurosci Abstr 9:610
Kuo DC, Hisamitsu T, de Groat WC (1984) A sympathetic projection from sacral paravertebral ganglia to the pelvic nerve and to postganglionic nerves on the surface of the urinary bladder and large intestine of the cat. J Comp Neurol 226:76–86
Kura H, Obara K, Yabu H (1992) Contractile responses to electrical field stimulation and ATP in guinea-pig urinary bladder. Comp Biochem Physiol C 102:193–197
Labadia A, Rivera L, Costa G, Garcia-Sacristan A (1988) Influence of the autonomic nervous system in the horse urinary bladder. Res Vet Sci 44:282–285
Lai HH, Munoz A, Smith CP, Boone TB, Somogyi GT (2011) Plasticity of non-adrenergic non-cholinergic bladder contractions in rats after chronic spinal cord injury. Brain Res Bull 86:91–96
Laird JM, Roza C, Cervero F (1996) Spinal dorsal horn neurons responding to noxious distension of the ureter in anesthetized rats. J Neurophysiol 76:3239–3248
Lambrecht G, Friebe T, Grimm U, Windscheif U, Bungardt E, Hildebrandt C, Bäumert HG, Spatz-Kümbel G, Mutschler E (1992) PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur J Pharmacol 217:217–219
Langley KN, Anderson HK (1895) The innervation of the pelvic and adjoining viscera: Part II. The bladder. J Physiol 19:71–84
Lantéri-Minet M, Bon K, de Pommery J, Michiels JF, Menétrey D (1995) Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res 105:220–232
Latini JM, Giannantoni A (2011) Pharmacotherapy of overactive bladder: epidemiology and pathophysiology of overactive bladder. Expert Opin Pharmacother 12:1017–1027
Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G (2000) Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol 278:F540–F553
Lawrence GW, Aoki KR, Dolly JO (2010) Excitatory cholinergic and purinergic signaling in bladder are equally susceptible to botulinum neurotoxin a consistent with co-release of transmitters from efferent fibers. J Pharmacol Exp Ther 334:1080–1086
Lazzeri M (2006) The physiological function of the urothelium — more than a simple barrier. Urol Int 76:289–295
Lee H-Y, Bardini M, Burnstock G (2000) Distribution of P2X receptors in the urinary bladder and ureter of the rat. J Urol 163:2002–2007
Lee WC, Chiang PH, Tain YL, Wu CC, Chuang YC (2012) Sensory dysfunction of bladder mucosa and bladder oversensitivity in a rat model of metabolic syndrome. PLoS One 7:e45578
Lee JG, Wein AJ, Levin RM (1994) Comparative pharmacology of the male and female rabbit bladder neck and urethra: involvement of nitric oxide. Pharmacology 48:250–259
Levin RM, Brendler K, Wein AJ (1983) Comparative pharmacological response of an in vitro whole bladder preparation (rabbit) with response of isolated smooth muscle strips. J Urol 130:377–381
Levin RM, Jacoby R, Wein AJ (1983) High-affinity, divalent ion-specific binding of 3H-ATP to homogenate derived from rabbit urinary bladder. Comparison with divalent-ion ATPase activity. Mol Pharmacol 23:1–7
Levin RM, Longhurst PA, Kato K, McGuire EJ, Elbadawi A, Wein AJ (1990) Comparative physiology and pharmacology of the cat and rabbit urinary bladder. J Urol 143:848–852
Levin RM, Malkowicz SB, Jacobowitz D, Wein AJ (1981) The ontogeny of the autonomic innervation and contractile response of the rabbit urinary bladder. J Pharmacol Exp Ther 219:250–257
Levin RM, Ruggieri MR, Velagapudi S, Gordon D, Altman B, Wein AJ (1986) Relevance of spontaneous activity to urinary bladder function: an in vitro and in vivo study. J Urol 136:517–521
Levin RM, Ruggieri MR, Wein AJ (1986) Functional effects of the purinergic innervation of the rabbit urinary bladder. J Pharmacol Exp Ther 236:452–457
Levin RM, Shofer FS, Wein AJ (1980) Estrogen-induced alterations in the autonomic responses of the rabbit urinary bladder. J Pharmacol Exp Ther 215:614–618
Levin RM, Tong Y-C, Wein AJ (1991) Effect of pregnancy on the autonomic response of the rabbit urinary bladder. Neurourol Urodyn 10:313
Levin RM, Wein AJ (1982) Response of the in vitro whole bladder (rabbit) preparation to autonomic agonists. J Urol 128:1087–1090
Levin RM, Zderic SA, Ewalt DH, Duckett JW, Wein AJ (1991) Effects of pregnancy on muscarinic receptor density and function in the rabbit urinary bladder. Pharmacology 43:69–77
Lewis SA, Lewis JR (2006) Kinetics of urothelial ATP release. Am J Physiol Renal Physiol 291:F332–F340
Li C, Peoples RW, Weight FF (1998) Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br J Pharmacol 123:1–3
Li Y, Xue L, Miao Q, Mao F, Yao L, Yuan J, Qin W, Zhao Y, Sun H, Liu F, Wang H (2013) Expression and electrophysiological characteristics of P2X3 receptors in interstitial cells of Cajal in rats with partial bladder outlet obstruction. BJU Int 111:843–851
Li JH, Yasay GD, Kau ST, Ohnmacht CJ, Trainor DA, Bonev AD, Heppner TJ, Nelson MT (1996) Studies of the KATP channel opening activity of the new dihydropyridine compound 9-(3-cyanophenyl)-3,4,6,7,9,10-hexahydro-1,8-(2H,5H)-acridinedione in bladder detrusor in vitro. Arzneimittelforschung 46:525–530
Lin MJ, Liu S-H, Lin-Shiau S-Y (1998) Phorbol ester-induced contractions of mouse detrusor muscle are inhibited by nifedipine. Naunyn Schmiedebergs Arch Pharmacol 357:553–557
Lincoln J, Burnstock G (1993) Autonomic innervation of the urinary bladder and urethra. In: Maggi C (ed) The autonomic nervous system, vol. 3. Nervous control of the urogenital system. Harwood Academic Publishers, Chur, pp 33–68
Lincoln J, Crockett M, Haven AJ, Burnstock G (1984) Rat bladder in the early stages of streptozotocin-induced diabetes: adrenergic and cholinergic innervation. Diabetologia 26:81–87
Lincoln J, Haven AJ, Sawyer M, Burnstock G (1984) The smooth muscle of rat bladder in the early stages of streptozotocin-induced diabetes. Br J Urol 56:24–30
Liu G, Daneshgari F (2005) Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol 288:F1220–F1226
Liu H, Jiang Y, Kuo H (2013) Increased suburothelial nerve fiber and purinergic P2X3 receptor expressions in patients with idiopathic detrusor overactivity and their relationship with botulinum toxin A therapeutic outcome. Neurourol Urodyn 32:733–735
Liu G, Li M, Daneshgari F (2008) Temporal expression of muscarinic and purinergic receptors in diabetic rat bladder. Neurourol Urodyn 27:594–595
Liu S-H, Lin-Shiau S-Y (1996) The effects of uranyl ions on neuromuscular transmission in the urinary bladder of the normal and streptozotocin-diabetic mouse. Naunyn Schmiedebergs Arch Pharmacol 354:773–778
Liu SH, Lin-Shiau SY (2000) Protein kinase C regulates purinergic component of neurogenic contractions in mouse bladder. J Urol 164:1764–1767
Liu F, Takahashi N, Yamaguchi O (2009) Expression of P2X3 purinoceptors in suburothelial myofibroblasts of the normal human urinary bladder. Int J Urol 16:570–575
Liu M, Xu YF, Feng Y, Yang FQ, Luo J, Zhai W, Che JP, Wang GC, Zheng JH (2013) Epigallocatechin gallate attenuates interstitial cystitis in human bladder urothelium cells by modulating purinergic receptors. J Surg Res 183:397–404
Lluel P, Barras M, Palea S (2002) Cholinergic and purinergic contribution to the micturition reflex in conscious rats with long-term bladder outlet obstruction. Neurourol Urodyn 21:142–153
Longhurst PA, Belis JA (1986) Abnormalities of rat bladder contractility in streptozotocin-induced diabetes mellitus. J Pharmacol Exp Ther 238:773–777
Longhurst PA, Belis JA, O'Donnell JP, Galie JR, Westfall DP (1984) A study of the atropine-resistant component of the neurogenic response of the rabbit urinary bladder. Eur J Pharmacol 99:295–302
Longhurst PA, Brotcke TP, Leggett RE, Levin RM (1992) The influence of streptozotocin-induced diabetes mellitus on the sensitivity of rat urinary bladder body and base strips to changes in extracellular calcium. Gen Pharmacol 23:83–88
Longhurst PA, Kang J, Wein AJ, Levin RM (1990) The influence of intravesical volume upon contractile responses of the whole bladder preparation from streptozotocin-diabetic rats. Gen Pharmacol 21:687–692
Luheshi G, Zar A (1990) Purinoceptor desensitization impairs but does not abolish the non-cholinergic motor transmission in rat isolated urinary bladder. Eur J Pharmacol 185:203–208
Luheshi GN, Zar MA (1990) Presence of non-cholinergic motor transmission in human isolated bladder. J Pharm Pharmacol 42:223–224
Luheshi GN, Zar MA (1990) Inhibitory effect of streptozotocin-induced diabetes on non-cholinergic motor transmission in rat detrusor and its prevention by sorbinil. Br J Pharmacol 101:411–417
Luheshi GN, Zar MA (1991) The effect of streptozotocin-induced diabetes on cholinergic motor transmission in the rat urinary bladder. Br J Pharmacol 103:1657–1662
Lukacsko P, Krell RD (1981) The effects of nucleotides on the response of the isolated guinea pig urinary bladder to nonadrenergic, noncholinergic nerve stimulation. Can J Physiol Pharmacol 59:1199–1201
Lukacsko P, Krell RD (1982) Response of the guinea-pig urinary bladder to purine and pyrimidine nucleotides. Eur J Pharmacol 80:401–406
Lundin A, Hallander H, Kallner A, Lundin UK, Österberg E (1989) Bacteriuria testing by the ATP method as an integral part in the diagnosis and therapy of urinary tract infection (UTI). J Biolumin Chemilumin 4:381–389
Ma L, Feugang JM, Konarski P, Wang J, Lu J, Fu S, Ma B, Tian B, Zou C, Wang Z (2006) Growth inhibitory effects of quercetin on bladder cancer cell. Front Biosci 11:2275–2285
MacDermott AB, Role LW, Siegelbaum SA (1999) Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci 22:443–485
Mackenzie I, Burnstock G (1984) Neuropeptide action on the guinea-pig bladder; a comparison with the effects of field stimulation and ATP. Eur J Pharmacol 105:85–94
Mackenzie I, Burnstock G, Dolly JO (1982) The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience 7:997–1006
Maggi CA (1991) Omega conotoxin and prejunctional modulation of the biphasic response of the rat isolated urinary bladder to single pulse electrical field stimulation. J Auton Pharmacol 11:295–304
Maggi CA (1993) The dual sensory and efferent function of capsaicin-sensitive primary sensory nerves in the bladder and urethra. In: Maggi C (ed) The autonomic nervous system, vol. 3. Nervous control of the urogenital system. Harwood Academic Publishers, Chur, pp 383–422
Maggi CA, Conte B, Furio M, Santicioli P, Giuliani S, Meli A (1989) Further studies on mechanisms regulating the voiding cycle of the rat urinary bladder. Gen Pharmacol 20:833–838
Maggi CA, Grimaldi G, Meli A (1982) The effects of nifedipine and verapamil on spontaneous and carbachol-stimulated contractions of rat urinary bladder "in vivo". Arch Int Pharmacodyn Ther 257:288–294
Maggi CA, Manzini S, Parlani M, Conte B, Giuliani S, Meli A (1988) The effect of nifedipine on spontaneous, drug-induced and reflexly-activated contractions of the rat urinary bladder: evidence for the participation of an intracellular calcium store to micturition contraction. Gen Pharmacol 19:73–81
Maggi CA, Santicioli P, Manzini S, Conti S, Giuliani S, Patacchini R, Meli A (1989) Functional studies on the cholinergic and sympathetic innervation of the rat proximal urethra: effect of pelvic ganglionectomy or experimental diabetes. J Auton Pharmacol 9:231–241
Maggi CA, Santicioli P, Meli A (1984) Postnatal development of myogenic contractile activity and excitatory innervation of rat urinary bladder. Am J Physiol 247:R972–R978
Maggi CA, Santicioli P, Meli A (1985) Pharmacological evidence for the existence of two components in the twitch response to field stimulation of detrusor strips from the rat urinary bladder. J Auton Pharmacol 5:221–229
Mansfield K, Cheng Y, Moore K (2012) Treatment of urothelial cells with lipopolysaccharide from enteropathogenic E. coli reduces stretch induced ATP release. J Urol 187:e368
Marchenko SM, Volkova TM, Fedorov OI (1987) ATP-activated ion conductance in isolated smooth muscle cells of the urinary bladder of the guinea pig. Neirofiziologiia 19:95–100
Marti-Cabrera M, Llopis P, Abengochea A, Ortiz JL, Climent VJ, Cortijo J, Morcillo EJ (1994) Effects of Ca2+ channel antagonists and benzodiazepine receptor ligands in normal and skinned rat urinary bladder. Eur J Pharmacol 255:157–165
Martin RS, Luong LA, Welsh NJ, Eglen RM, Martin GR, MacLennan SJ (2000) Effects of cannabinoid receptor agonists on neuronally-evoked contractions of urinary bladder tissues isolated from rat, mouse, pig, dog, monkey and human. Br J Pharmacol 129:1707–1715
Martins JP, Silva RB, Coutinho-Silva R, Takiya CM, Battastini AM, Morrone FB, Campos MM (2012) The role of P2X7 purinergic receptors in inflammatory and nociceptive changes accompanying cyclophosphamide-induced haemorrhagic cystitis in mice. Br J Pharmacol 165:183–196
Mastri AR (1980) Neuropathology of diabetic neurogenic bladder. Ann Intern Med 92:316–318
Masuda N, Uchida W, Shirai Y, Shibasaki K, Goto K, Takenaka T (1995) Effect of the potassium channel opener YM934 on the contractile response to electrical field stimulation in pig detrusor smooth muscle. J Urol 154:1914–1920
Matsumoto-Miyai K, Kagase A, Murakawa Y, Momota Y, Kawatani M (2009) Extracellular Ca2+ regulates the stimulus-elicited ATP release from urothelium. Auton Neurosci 150:94–99
Matsumoto-Miyai K, Yamada E, Yoshizumi M, Kawatani M (2012) The regulation of distention-induced ATP release from urothelium by the adenylyl cyclase-cyclic AMP pathway. Biomed Res 33:153–157
Matsumura S, Taira N, Hashimoto K (1968) The pharmacological behaviour of the urinary bladder and its vasculature of the dog. Tohoku J Exp Med 96:247–258
Mattiasson A, Andersson K-E, Andersson PO, Larsson B, Sjögren C, Uvelius B (1990) Nerve-mediated functions in the circular and longitudinal muscle layers of the proximal female rabbit urethra. J Urol 143:155–160
McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ (2009) Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J Urol 181:1459–1466
McCloskey KD (2011) Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol 202:233–254
McCloskey KD, Anderson UA, Davidson RA, Bayguinov YR, Sanders KM, Ward SM (2009) Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/W v mice. Br J Pharmacol 156:273–283
McDermott C, Chess-Williams R, Grant GD, Perkins AV, McFarland AJ, Davey AK, Anoopkumar-Dukie S (2012) Effects of Pseudomonas aeruginosa virulence factor pyocyanin on human urothelial cell function and viability. J Urol 187:1087–1093
McDonald WF, White TD (1984) Adenosine released from synaptosomes is derived from the extracelluar dephosphonylation of released ATP. Prog Neuropsychopharmacol Biol Psychiatr 8:487–494
McGuire EJ, Herlihy E (1978) Bladder and urethral responses to isolated sacral motor root stimulation. Invest Urol 16:219–223
McMurray G, Dass N, Brading AF (1998) Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. Br J Pharmacol 123:1579–1586
Meldrum LA, Burnstock G (1985) Evidence against VIP or substance P being the transmitter in non-cholinergic excitatory nerves supplying the guinea-pig bladder. J Pharm Pharmacol 37:432–434
Meng E, Chang HY, Chang SY, Sun GH, Yu DS, Cha TL (2011) Involvement of purinergic neurotransmission in ketamine induced bladder dysfunction. J Urol 186:1134–1141
Meng E, Lin WY, Lee WC, Chuang YC (2012) Pathophysiology of overactive bladder. LUTS 4:48–55
Meng E, Young JS, Brading AF (2008) Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn 27:79–87
Messori E, Rizzi CA, Candura SM, Lucchelli A, Balestra B, Tonini M (1995) 5-Hydroxytryptamine receptors that facilitate excitatory neuromuscular transmission in the guinea-pig isolated detrusor muscle. Br J Pharmacol 115:677–683
Michel AD, Lundström K, Buell GN, Surprenant A, Valera S, Humphrey PP (1996) The binding characteristics of a human bladder recombinant P2X purinoceptor, labelled with [3H]α-meATP, [35S]-ATPγS or [33P]-ATP. Br J Pharmacol 117:1254–1260
Milicic I, Buckner SA, Daza A, Coghlan M, Fey TA, Brune ME, Gopalakrishnan M (2006) Pharmacological characterization of urinary bladder smooth muscle contractility following partial bladder outlet obstruction in pigs. Eur J Pharmacol 532:107–114
Miller H, Simpson CA, Yeates WK (1965) Bladder dysfunction in multiple sclerosis. Br Med J 1:1265–1269
Mitchell ME, Gonzales R, Cabral BH, Bauer SB, Gearhart JP, Filmer RB (1987) Bladder augmentation problems in neurovesical dysfunction. Dialogues Ped Urol 10:1
Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M (2009) The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284:21257–21264
Mohlin C, Säve S, Nilsson M, Persson K (2009) Studies of the extracellular ATP–adenosine pathway in human urinary tract epithelial cells. Pharmacology 84:196–202
Moon HS, Lee JW, Park SY, Son YW, Kim YT (2010) Oxybutynin and propiverine suppress adenosine triphosphate-induced bladder overactivity other than through antimuscarinic mechanisms. Urology 76:509–512
Moore KH, Gilpin SA, Dixon JS, Richmond DH, Sutherst JR (1992) Increase in presumptive sensory nerves of the urinary bladder in idiopathic detrusor instability. Br J Urol 70:370–372
Moore KH, Ray FR, Barden JA (2001) Loss of purinergic P2X3 and P2X5 receptor innervation in human detrusor from adults with urge incontinence. J Neurosci 21(RC166):1–6
Moreland RB, Brioni JD, Sullivan JP (2004) Emerging pharmacologic approaches for the treatment of lower urinary tract disorders. J Pharmacol Exp Ther 308:797–804
Morgan C, de Groat WC, Nadelhaft I (1986) The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol 243:23–40
Moro C, Milligan C, Leeds C, Chess-Williams R (2009) Spontaneous contractile activity of the urothelium is increased by muscarinic and purinergic receptor stimulation. Neurourol Urodyn 28:867–868
Morris JL, Gibbins IL (1992) Co-transmission and neuromodulation. In: Burnstock G, Hoyle CHV (eds) Autonomic neuroeffector mechanisms. Harwood Academic Publishers, Chur, pp 33–119
Moss HE, Burnstock G (1985) A comparative study of electrical field stimulation of the guinea-pig, ferret and marmoset urinary bladder. Eur J Pharmacol 114:311–316
Moss HE, Lincoln J, Burnstock G (1987) A study of bladder dysfunction during streptozotocin-induced diabetes in the rat using an in vitro whole bladder preparation. J Urol 138:1279–1284
Moss HE, Tansey EM, Burnstock G (1989) Abnormalities of responses to autonomic stimulation in the mouse urinary bladder associated with Semliki Forest virus-induced demyelination. J Urol 142:850–854
Müller DP, Goss-Sampson MA (1990) Neurochemical, neurophysiological, and neuropathological studies in vitamin E deficiency. Crit Rev Neurobiol 5:239–263
Mumtaz FH, Lau DH, Siddiqui EJ, Morgan RJ, Thompson CS, Mikhailidis DP (2006) Changes in cholinergic and purinergic neurotransmission in the diabetic rabbit bladder. In Vivo 20:1–4
Munoz A, Gangitano D, Smith CP, Boone TB, Somogyi GT (2009) Urothelium is the primary source of ATP and NO release in the rat urinary bladder: a novel method for an urothelium free preparation. J Urol 181:147
Munoz A, Romain Z, Munch E, Gangitano D, Boone T, Smith C, Somogyi G (2008) Changes in purinergic and nitrergic sensory signals in female rats during early diabetes. Neurourol Urodyn 28:110–111
Munoz A, Somogyi GT, Boone TB, Ford AP, Smith CP (2012) Modulation of bladder afferent signals in normal and spinal cord-injured rats by purinergic P2X3 and P2X2/3 receptors. BJU Int 110:E409–E414
Munoz A, Somogyi GT, Boone TB, Smith CP (2011) Lumbosacral sensory neuronal activity is enhanced by activation of urothelial purinergic receptors. Brain Res Bull 86:380–384
Murakami S, Yoshida M, Masunaga K, Maeda Y, Ueda S (2008) Change in acetylcholine release from rat bladder with partial outlet obstruction. BJU Int 101:633–639
Nakagomi H, Mochizuki T, Miyamoto T, Kira S, Yoshiyama M, Araki I, Koizumi S, Moriyama Y, Takeda M (2011) VNUT plays an important role in vesicular storage and subsequent exocytosis of ATP from bladder epithelium upon mechanical stretch stimulation. Neurourol Urodyn 30:1181–1182
Nakagomi H, Mochizuki T, Miyamoto T, Yoshiyama M, Araki I, Moriyama Y, Koizumi S, Takeda M (2012) VNUT (vesicular nucleotide transporter) plays a crucial role in stretch-evoked ATP release from urothelium. J Urol 187:e203
Nakayama S (1993) Effects of excitatory neurotransmitters on Ca2+ channel current in smooth muscle cells isolated from guinea-pig urinary bladder. Br J Pharmacol 110:317–325
Namasivayam S, Eardley I, Morrison JFB (1999) Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int 84:854–860
Nance DM, Burns J, Klein CM, Burden HW (1988) Afferent fibers in the reproductive system and pelvic viscera of female rats: anterograde tracing and immunocytochemical studies. Brain Res Bull 21:701–709
Naramatsu M, Yamashita T, Kokubun S (1997) The signalling pathway which causes contraction via P2-purinoceptors in rat urinary bladder smooth muscle. Br J Pharmacol 122:558–562
Nazif O, Teichman JM, Gebhart GF (2007) Neural upregulation in interstitial cystitis. Urology 69:24–33
Needleman P, Minkes MS, Douglas JR (1974) Stimulation of prostaglandin biosynthesis by adenine nucleotides. Profile of prostaglandin release by perfused organs. Circ Res 34:455–460
Nergårdh A, Kinn AC (1983) Neurotransmission in activation of the contractile response in the human urinary bladder. Scand J Urol Nephrol 17:153–157
Neuhaus J, Pfeiffer F, Wolburg H, Horn LC, Dorschner W (2005) Alterations in connexin expression in the bladder of patients with urge symptoms. BJU Int 96:670–676
Nicholls J, Hourani SMO, Kitchen I (1990) The ontogeny of purinoceptors in rat urinary bladder and duodenum. Br J Pharmacol 100:874–878
Nicholls J, Hourani SMO, Kitchen I (1992) Characterization of P1-purinoceptors on rat duodenum and urinary bladder. Br J Pharmacol 105:639–642
Nicholls J, Hourani SMO, Kitchen I (1992) Degradation of extracellular adenosine and ATP by adult and neonate rat duodenum and urinary bladder. Pharmacol Commun 2:203–210
Nile CJ, de Vente J, Gillespie JI (2010) Stretch independent regulation of prostaglandin E2 production within the isolated guinea-pig lamina propria. BJU Int 105:540–548
Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H, Yoshimura N (2005) Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology 66:1332–1337
Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N (2004) Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci 20:474–482
Nishijima S, Sugaya K, Kadekawa K, Naka H, Miyazato M (2009) Comparison of the effect of anti-muscarinic agents on bladder activity, urinary ATP level, and autonomic nervous system in rats. Biomed Res 30:107–112
Nishimura T, Akasu T (1994) Endogenous ATP modulates nicotinic transmission through presynaptic P2 receptors in rabbit parasympathetic ganglia. Neurosci Res 19:S31
Nishimura T, Tokimasa T (1996) Purinergic cation channels in neurons of rabbit vesical parasympathetic ganglia. Neurosci Lett 212:215–217
Noto H, Roppolo JR, Steers WD, de Groat WC (1989) Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res 492:99–115
Nunn PA, Newgreen DT (1999) An investigation into the bladder responses induced via pelvic nerve stimulation in the anaesthetised rat. Br J Pharmacol 126:227P
Obara K, Lepor H, Walden PD (1998) Localization of P 2Y1 purinoceptor transcripts in the rat penis and urinary bladder. J Urol 160:587–591
Ochodnicky P, Michel MB, Butter JJ, Seth J, Panicker JN, Michel MC (2013) Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol Res 70:147–154
Ohnishi N, Park YC, Kurita T, Kajimoto N (1997) Role of ATP and related purine compounds on urethral relaxation in male rabbits. Int J Urol 4:191–197
Oike M, Creed KE, Onoue H, Tanaka H, Ito Y (1998) Increase in calcium in smooth muscle cells of the rabbit bladder induced by acetylcholine and ATP. J Auton Nerv Syst 69:141–147
Olsen SM, Stover JD, Nagatomi J (2011) Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng 39:688–697
O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, McMahon SB (2001) A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol 165:1730–1734
O'Reilly BA, Kosaka AH, Knight GE, Chang TK, Ford APDW, Rymer JM, Popert R, Burnstock G, McMahon SB (2002) P2X receptors and their role in female idiopathic detrusor instability. J Urol 167:157–164
Ouslander JG (2004) Management of overactive bladder. N Engl J Med 350:786–799
Owen SJ, Massa HH, Rose'Meyer RB (2012) Loss of adenosine A2B receptor mediated relaxant responses in the aged female rat bladder; effects of dietary phytoestrogens. Naunyn Schmiedebergs Arch Pharmacol 385:539–549
Palea S, Artibani W, Ostardo E, Trist DG, Pietra C (1993) Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol 150:2007–2012
Palea S, Corsi M, Pietra C, Artibani W, Calpista A, Gaviraghi G, Trist DG (1994) ADPβS induces contraction of the human isolated urinary bladder through a purinoceptor subtype different from P2X and P2Y. J Pharmacol Exp Ther 269:193–197
Palea S, Pietra C, Trist DG, Artibani W, Calpista A, Corsi M (1995) Evidence for the presence of both pre- and postjunctional P2-purinoceptor subtypes in human isolated urinary bladder. Br J Pharmacol 114:35–40
Pandita RK, Andersson KE (2002) Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168:1230–1234
Pannek J, Janek S, Sommerer F, Tannapfel A (2009) Expression of purinergic P2X2-receptors in neurogenic bladder dysfunction due to spinal cord injury: a preliminary immunohistochemical study. Spinal Cord 47:561–564
Parija SC, Raviprakash V, Mishra SK (1991) Adenosine- and α, β-methylene ATP-induced differential inhibition of cholinergic and non-cholinergic neurogenic responses in rat urinary bladder. Br J Pharmacol 102:396–400
Park Y-C, Sugiyama T, Kaneko S, Kurita T (1986) Sympathetic contribution to bladder outlet obstructions: quantitative analysis of tissue catecholamine content. Neurourol Urodyn 5:573–577
Paro M, Italiano G, Travagli RA, Petrelli L, Zanoni R, Prosdocimi M, Fiori MG (1990) Cystometric changes in alloxan diabetic rats: evidence for functional and structural correlates of diabetic autonomic neuropathy. J Auton Nerv Syst 30:1–11
Paro M, Prosdocimi M (1987) Experimental diabetes in the rat: alterations in the vesical function. J Auton Nerv Syst 21:59–66
Paro M, Prosdocimi M, Zhang WX, Sutherland G, Sima AA (1989) Autonomic neuropathy in BB rats and alterations in bladder function. Diabetes 38:1023–1030
Partanen M, Santer RM, Hervonen A (1980) The effect of ageing on the histochemically demonstrable catecholamines in the hypogastric (main pelvic) ganglion of the rat. Histochem J 12:527–535
Patra PB, Westfall DP (1994) Potentiation of purinergic neurotransmission in guinea pig urinary bladder by histamine. J Urol 151:787–790
Patra PB, Westfall DP (1996) Potentiation by bradykinin and substance P of purinergic neurotransmission in urinary bladder. J Urol 156:532–535
Persson CGA (1976) Inhibitory effect at the bladder–urethral junction. Acta Physiol Scand 97:139–141
Persson K, Andersson K-E (1992) Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol 106:416–422
Pertwee RG, Fernando SR (1996) Evidence for the presence of cannabinoid CB1 receptors in mouse urinary bladder. Br J Pharmacol 118:2053–2058
Peterson JS, Noronha-Blob L (1989) Effects of selective cholinergic antagonists and α, β-methylene ATP on guinea-pig urinary bladder contractions in vivo following pelvic nerve stimulation. J Auton Pharmacol 9:303–313
Piechota HJ, Dahms SE, Nunes LS, Dahiya R, Lue TF, Tanagho EA (1998) In vitro functional properties of the rat bladder regenerated by the bladder acellular matrix graft. J Urol 159:1717–1724
Pinna C, Bolego C, Puglisi L (1994) Effect of substance P and capsaicin on urinary bladder of diabetic rats and the role of the epithelium. Eur J Pharmacol 271:151–158
Pinna C, Glass R, Knight G, Bolego C, Puglisi L, Burnstock G (2005) Purine- and pyrimidine-induced responses and P2Y receptor characterisation in the hamster proximal urethra. Br J Pharmacol 144:510–518
Pinna C, Knight G, Puglisi L, Burnstock G (1998) Neurogenic and non-neurogenic responses in the urinary bladder of hibernating hamster. Br J Pharmacol 123:1281–1287
Pinna C, Puglisi L, Burnstock G (1998) ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolated proximal urethra. Br J Pharmacol 124:1069–1074
Pinna C, Sanvito P, Puglisi L (2006) Altered neurogenic and mechanical responses to acetylcholine, ATP and substance P in detrusor from rat with outlet obstruction. Life Sci 79:1301–1306
Pinna C, Ventura S, Puglisi L, Burnstock G (1996) A pharmacological and histochemical study of hamster urethra and the role of urothelium. Br J Pharmacol 119:655–662
Pinna C, Zanardo R, Cignarella A, Bolego C, Eberini I, Nardi F, Zancan V, Puglisi L (2000) Diabetes influences the effet of 17β-estradiol on mechanical responses of rat urethra and detrusor strips. Life Sci 6:617–627
Pinna C, Zanardo R, Puglisi L (2000) Prostaglandin-release impairment in the bladder epithelium of streptozotocin-induced diabetic rats. Eur J Pharmacol 388:267–273
Pittam BS, Burnstock G, Purves RD (1987) Urinary bladder intramural neurones: an electrophysiological study utilizing a tissue culture preparation. Brain Res 403:267–278
Prakasam HS, Herrington H, Roppolo JR, Jackson EK, Apodaca G (2012) Modulation of bladder function by luminal adenosine turnover and A1 receptor activation. Am J Physiol Renal Physiol 303:F279–F292
Probst M, Dahiya R, Carrier S, Tanagho EA (1997) Reproduction of functional smooth muscle tissue and partial bladder replacement. Br J Urol 79:505–515
Prosdocimi M, Paro M (1990) Urinary bladder innervation in experimental diabetes. J Auton Nerv Syst 30:S123–S127
Qayyum MA, Fatani JA, Abbas MO (1989) Degeneration of adrenergic nerves in the urinary bladder during pregnancy. Acta Anat (Basel) 136:303–305
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Rapp DE, Lyon MB, Bales GT, Cook SP (2005) A role for the P2X receptor in urinary tract physiology and in the pathophysiology of urinary dysfunction. Eur Urol 48:303–308
Rapp DE, Turk KW, Bales GT, Cook SP (2006) Botulinum toxin type A inhibits calcitonin gene-related peptide release from isolated rat bladder. J Urol 175:1138–1142
Ratz PH, McCammon KA, Altstatt D, Blackmore PF, Shenfeld OZ, Schlossberg SM (1999) Differential effects of sex hormones and phytoestrogens on peak and steady state contractions in isolated rabbit detrusor. J Urol 162:1821–1828
Ribeiro JA (1979) Purinergic modulation of transmitter release. J Theor Biol 80:259–270
Risholm L (1954) Studies on renal colic and its treatment by posterior splanchnic block. Acta Chir Scand Suppl 184:5–64
Rocha I, Burnstock G, Spyer KM (2001) Effect on urinary bladder function and arterial blood pressure of the activation of putative purine receptors in brainstem areas. Auton Neurosci 88:6–15
Rockenbach L, Bavaresco L, Fernandes FP, Cappellari AR, Barrios CH, Bueno MF, Oliveira Battastini AM (2013) Alterations in the extracellular catabolism of nucleotides are involved in the antiproliferative effect of quercetin in human bladder cancer T24 cells. Urol Oncol 31:1204–1211
Rong W, Burnstock G (2004) Activation of ureter nociceptors by exogenous and endogenous ATP in guinea pig. Neuropharmacology 47:1093–1101
Rong W, Spyer M, Burnstock G (2002) Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol 541:591–600
Roza C, Laird JM, Cervero F (1998) Spinal mechanisms underlying persistent pain and referred hyperalgesia in rats with an experimental ureteric stone. J Neurophysiol 79:1603–1612
Ruggieri MR (2006) Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol 3:206–215
Ruggieri MR, Whitmore KE, Levin RM (1990) Bladder purinergic receptors. J Urol 144:176–181
Ryhammer AM, Bek KM, Laurberg S (1995) Multiple vaginal deliveries increase the risk of permanent incontinence of flatus urine in normal premenopausal women. Dis Colon Rectum 38:1206–1209
Sadananda P, Kao FC, Liu L, Mansfield KJ, Burcher E (2012) Acid and stretch, but not capsaicin, are effective stimuli for ATP release in the porcine bladder mucosa: are ASIC and TRPV1 receptors involved? Eur J Pharmacol 683:252–259
Sadananda P, Mansfield KJ, Burcher E (2009) ATP release in response to acid and capsaicin provides evidence for sensory roles of vanilloid and asic receptor systems in the rat bladder mucosa. J Urol 181:147
Saito M, Gotoh M, Kato K, Kondo A (1991) Influence of aging on the rat urinary bladder function. Urol Int 47:39–42
Saito M, Kondo A, Kato T, Hasegawa S, Miyake K (1993) Response of the human neurogenic bladder to KCl, carbachol, ATP and CaCl2. Br J Urol 72:298–302
Saito M, Kondo A, Kato T, Levin RM (1993) Response of isolated human neurogenic detrusor smooth muscle to intramural nerve stimulation. Br J Urol 72:723–727
Saito M, Ohmura M, Kondo A (1996) Effects of long-term partial outflow obstruction on bladder function in the rat. Neurourol Urodyn 15:157–165
Salmon UJ, Walter RI, Geist SH (1941) The use of estrogens in the treatment of dysuria and incontinence in postmenopausal women. Am J Obstet Gynecol 42:845–851
Sann H, Cervero F (1988) Afferent innervation of the guinea-pig's ureter. Agents Actions 25:243–245
Sann H, Jancsó G, Ambrus A, Pierau F-K (1995) Capsaicin treatment induces selective sensory degeneration and increased sympathetic innervation in the rat ureter. Neuroscience 67:953–966
Sann H, Walb G, Pierau FK (1997) Postnatal development of the autonomic and sensory innervation of the musculature in the rat urinary bladder. Neurosci Lett 236:29–32
Santicioli P, Gamse R, Maggi CA, Meli A (1987) Cystometric changes in the early phase of streptozotocin-induced diabetes in rats: evidence for sensory changes not correlated to diabetic neuropathy. Naunyn Schmiedebergs Arch Pharmacol 335:580–587
Santicioli P, Maggi CA, Meli A (1986) The postganglionic excitatory innervation of the mouse urinary bladder and its modulation by prejunctional GABAB receptors. J Auton Pharmacol 6:53–66
Santoso AG, Sonarno IA, Arsad NA, Liang W (2010) The role of the urothelium and ATP in mediating detrusor smooth muscle contractility. Urology 76:1267–12
Säve S, Persson K (2010) Effects of adenosine A2A and A2B receptor activation on signaling pathways and cytokine production in human uroepithelial cells. Pharmacology 86:129–137
Säve S, Persson K (2010) Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun 78:3609–3615
Schaufele P, Schumacher E, Acevedo CG, Contreras E (1995) Diazepam, adenosine analogues and calcium channel antagonists inhibit the contractile activity of the mouse urinary bladder. Arch Int Pharmacodyn Ther 329:454–466
Schwartzman RJ (1993) Reflex sympathetic dystrophy. Curr Opin Neurol Neurosurg 6:531–536
Searl TJ, Silinsky EM (2012) Modulation of purinergic neuromuscular transmission by phorbol dibutyrate is independent of protein kinase C in murine urinary bladder. J Pharmacol Exp Ther 342:312–317
Semerdzhiev Y, Frederiksen H, Hedlund P, Davidsson T, Mansson W, Uvelius B (2006) Cystometric and in vitro muscle studies of cystoplastic appendiceal segments in the rat. Neurourol Urodyn 25:259–267
Senba E, Daddona PE, Nagy JI (1987) Development of adenosine deaminase-immunoreactive neurons in the rat brain. Brain Res 428:59–71
Shabbir M, Burnstock G (2009) Purinergic receptor-mediated effects of ATP in urogenital malignant diseases. Int J Urol 16:143–150
Shabbir M, Ryten M, Thompson CS, Mikhailidis DP, Burnstock G (2008) Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int 101:106–112
Shabir S, Cross W, Kirkwood L, Pearson JF, Appleby PA, Walker D, Eardley I, Southgate J (2013) Functional expression of purinergic P2 receptors and transient receptor potential channels by human urothelium. Am J Physiol - Renal Physiol 305:F396–406
Shafik A, El-Sibai O, Shafik AA, Shafik I (2004) Identification of interstitial cells of Cajal in human urinary bladder: concept of vesical pacemaker. Urology 64:809–813
Shinnick-Gallagher P, Gallagher JA, Griffith WH (1986) Inhibition in parasympathetic ganglia. In: Karczmar AG, Koketsu K, Nishi S (eds) Autonomic and enteric ganglia. Plenum, New York, pp 335–367
Shinozuka K, Bjur RA, Westfall DP (1990) Effects of α, β-methylene ATP on the prejunctional purinoceptors of the sympathetic nerves of the rat caudal artery. J Pharmacol Exp Ther 254:900–904
Sibley GNA (1984) A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol 354:431–443
Silinsky EM, Hubbard JI (1973) Release of ATP from rat motor nerve terminals. Nature 243:404–405
Silva J, Silva C, Saraiva L, Silva A, Pinto R, Dinis P, Cruz F (2008) Intraprostatic botulinum toxin type A injection in patients unfit for surgery presenting with refractory urinary retention and benign prostatic enlargement. Effect on prostate volume and micturition resumption. Eur Urol 53:153–159
Silva-Ramos M, Duarte-Araújo M, Silva I, Lafuente-De-Carvalho JM, Correia-De-Sá P (2009) Activation of P2X1 purinoceptors facilitate evoked acetylcholine release in human obstructed bladders. Eur Urol Suppl 8:189
Silva-Ramos M, Silva I, Oliveira O, Ferreira S, Reis MJ, Oliveira JC, Correia-de-Sá P (2013) Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS One 8:e64696
Silva-Ramos M, Silva I, Timoteo MA, Carneito I, Vieira C, Silva N, Monteiro D, Correia J, Ferreirinha F, Sévigny J, Correia-De-Sá P (2012) UDP-sensitive P2Y6 receptors play a dual role in the human urinary bladder indirectly via the release of ATP from urothelium. Neurourol Urodyn 31:1023
Sjögren C, Andersson KE (1979) Inhibition of ATP-induced contraction in the guinea-pig urinary bladder in vitro and in vivo. Acta Pharmacol Toxicol (Copenh) 44:221–227
Sjögren C, Andersson K-E (1979) Effects of cholinoceptor blocking drugs, adrenoceptor stimulants, and calcium antagonists on the transmurally stimulated guinea-pig urinary bladder in vitro and in vivo. Acta Pharmacol Toxicol 44:228–234
Sjögren C, Andersson K-E, Andersson PO, Mattiasson A, Uvelius B (1988) Different effects of neuropeptide Y on electrically induced contractions in the longitudinal and circular smooth muscle layers of the female rabbit urethra. Acta Physiol Scand 133:177–181
Sjögren C, Andersson K-E, Husted S, Mattiasson A, Møller-Madsen B (1982) Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol 128:1368–1371
Sjuve Scott R, Uvelius B, Arner A (2004) Changes in intracellular calcium concentration and P2X1 receptor expression in hypertrophic rat urinary bladder smooth muscle. Neurourol Urodyn 23:361–366
Sjuve R, Ingvarson T, Arner A, Uvelius B (1995) Effects of purinoceptor agonists on smooth muscle from hypertrophied rat urinary bladder. Eur J Pharmacol 276:137–144
Smith DJ, Chapple CR (1994) In vitro response of human bladder smooth muscle in unstable obstructed male bladders: a study of pathophysiological causes. Neurourol Urodyn 13:414–415
Smith CP, Gangitano DA, Munoz A, Salas NA, Boone TB, Aoki KR, Francis J, Somogyi GT (2008) Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int 52:1068–1075
Smith CP, Radziszewski P, Borkowski A, Somogyi GT, Boone TB, Chancellor MB (2004) Botulinum toxin A has antinociceptive effects in treating interstitial cystitis. Urology 64:871–875
Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT (2005) Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int 47:291–297
Sneddon P, Burnstock G (1984) Inhibition of excitatory junction potentials in guinea-pig vas deferens by α, β-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol 100:85–90
Sneddon P, McLees A (1992) Purinergic and cholinergic contractions in adult and neonatal rabbit bladder. Eur J Pharmacol 214:7–12
Snyder SH (1985) Adenosine as a neuromodulator. Annu Rev Neurosci 8:103–124
Sosnowski M, Yaksh TL (1990) The role of spinal and brainstem adenosine receptors in the modulation of the volume-evoked micturition reflex in the unanesthetized rat. Brain Res 515:207–213
Speakman MJ, Walmsley D, Brading AF (1988) An in vitro pharmacological study of the human trigone — a site of non-adrenergic, non-cholinergic neurotransmission. Br J Urol 61:304–309
Sperlágh B, Vizi ES (1991) Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem 56:1466–1470
Spitsbergen JM, Clemow DB, McCarty R, Steers WD, Tuttle JB (1998) Neurally mediated hyperactive voiding in spontaneously hypertensive rats. Brain Res 790:151–159
Stanton SL, Kerr-Wilson R, Harris VG (1980) The incidence of urological symptoms in normal pregnancy. Br J Obstet Gynaecol 87:897–900
Steers WD, Mackway-Gerardi AM, Ciambotti J, de Groat WC (1994) Alterations in neural pathways to the urinary bladder of the rat in response to streptozotocin-induced diabetes. J Auton Nerv Syst 47:83–94
Sterling K, Roth J, Laaris N, Chai T, Sun Y (2012) Rat and human bladder urothelial cells have decreased ATP-evoked increase in intracellular calcium with knockdown of P2Y2 receptors. J Urol 187:e107
Stewart FA (1986) Mechanism of bladder damage and repair after treatment with radiation and cytostatic drugs. Br J Cancer 53:280–291
Studeny S, Torabi A, Vizzard MA (2005) P2X2 and P2X3 receptor expression in postnatal and adult rat urinary bladder and lumbosacral spinal cord. Am J Physiol Regul Integr Comp Physiol 289:R1155–R1168
Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC (2009) Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int 103:1686–1693
Sugaya K, Nishijima S, Tasaki S, Kadekawa K, Miyazato M, Ogawa Y (2007) Effects of propiverine and naftopidil on the urinary ATP level and bladder activity after bladder stimulation in rats. Neurosci Lett 429:142–146
Sugaya K, Nishijima S, Tasaki S, Kadekawa K, Miyazato M, Ogawa Y (2008) Mechanisms by which a phytotherapeutic drug influences bladder activity in rats. J Urol 179:770–774
Sui GP, Wu C, Fry CH (2004) Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol 171:938–943
Sui GP, Wu C, Fry CH (2006) Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int 97:1327–1331
Sun Y, Chai TC (2002) Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU Int 90:381–385
Sun Y, Chai TC (2004) Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol 171:448–452
Sun Y, Chai TC (2006) Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290:C27–C34
Sun Y, Keay S, Deyne P, Chai T (2001) Stretch-activated release of adenosine triphosphate by bladder uroepithelia is augmented in interstitial cystitis. Urology 57:131
Sun Y, Keay S, Lehrfeld TJ, Chai TC (2009) Changes in adenosine triphosphate-stimulated ATP release suggest association between cytokine and purinergic signaling in bladder urothelial cells. Urology 74:1163–1168
Sun Y, MaLossi J, Jacobs SC, Chai TC (2002) Effect of doxazosin on stretch-activated adenosine triphosphate release in bladder urothelial cells from patients with benign prostatic hyperplasia. Urology 60:351–356
Suzuki H, Kokubun S (1994) Subtypes of purinoceptors in rat and dog urinary bladder smooth muscles. Br J Pharmacol 112:117–122
Syed N, Kennedy C (2012) Pharmacology of P2X receptors. WIREs Membr Transp Signal 1:16–30
Tagliani M, Candura SM, Di Nucci A, Franceschetti GP, D'Agostino G, Ricotti P, Fiori E, Tonini M (1997) A re-appraisal of the nature of the atropine-resistant contraction to electrical field stimulation in the human isolated detrusor muscle. Naunyn Schmiedebergs Arch Pharmacol 356:750–755
Taira N (1972) The autonomic pharmacology of the bladder. Annu Rev Pharmacol 12:197–208
Takahashi R, Yunoki T, Naito S, Yoshimura N (2012) Differential effects of botulinum neurotoxin A on bladder contractile responses to activation of efferent nerves, smooth muscles and afferent nerves in rats. J Urol 188:1993–1999
Takeda M, Lepor H (1995) Nitric oxide synthase in dog urethra: a histochemical and pharmacological analysis. Br J Pharmacol 116:2517–2523
Takeda M, Mochizuki T, Yoshiyama M, Nakagomi H, Kobayashi H, Sawada N, Zakohji H, Du S, Araki I (2010) Sensor mechanism and afferent signal transduction of the urinary bladder: special focus on transient receptor potential ion channels. LUTS 2:51–60
Tammela TL, Briscoe JA, Levin RM, Longhurst PA (1994) Factors underlying the increased sensitivity to field stimulation of urinary bladder strips from streptozotocin-induced diabetic rats. Br J Pharmacol 113:195–203
Tammela T, Kontturi M, Lukkarinen O (1986) Postoperative urinary retention: II. Micturition problems after the first catheterization. Scand J Urol Nephrol 20:257–260
Tammela T, Lasanen L, Waris T (1990) Effect of distension on adrenergic innervation of the rat urinary bladder. Urol Res 18:345–348
Tanaka I, Nagase K, Tanase K, Aoki Y, Akino H, Yokoyama O (2011) Modulation of stretch evoked adenosine triphosphate release from bladder epithelium by prostaglandin E2. J Urol 185:341–346
Tanner R, Chambers P, Khadra MH, Gillespie JI (2000) The production of nerve growth factor by human bladder smooth muscle cells in vivo and in vitro. BJU Int 85:1115–1119
Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR (2004) P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93:1344–1348
Theobald RJ Jr (1982) Arylazido aminopropionyl ATP (ANAPP3) antagonism of cat urinary bladder contractions. J Auton Pharmacol 2:175–179
Theobald RJ Jr (1983) The effect of arylazido aminopropionyl ATP on atropine resistant contractions of the cat urinary bladder. Life Sci 32:2479–2484
Theobald RJ Jr (1983) Evidence against purinergic nerve fibres in the hypogastric nerves of the cat. J Auton Pharmacol 3:235–239
Theobald RJ Jr (1986) Changes in ureteral peristaltic activity induced by various stimuli. Neurourol Urodyn 5:493–505
Theobald RJ Jr (1992) Subclasses of purinoceptors in feline bladder. Eur J Pharmacol 229:125–130
Theobald RJ Jr (1995) Purinergic and cholinergic components of bladder contractility and flow. Life Sci 56:445–454
Theobald RJ Jr, de Groat WC (1989) The effects of purine nucleotides on transmission in vesical parasympathetic ganglia of the cat. J Auton Pharmacol 9:167–182
Theobald RJ Jr, Hoffman V (1986) Long-lasting blockade of P2-receptors of the urinary bladder in vivo following photolysis of arylazido aminopropionyl ATP, a photoaffinity label. Life Sci 38:1591–1595
Theobald RJ Jr, Zepp EA, Westhoff R (1988) Endocrine influences on the detrusor of male and female cats. Neurourol Urodynam 7:493–500
Thi M, Melman A, Spray DC, Suadicani S (2011) Pannexin-1 channels provide a pathway for ATP release from rat bladder mucosa. Neurourol Urodyn 30:235–236
Thiruchelvam N, Wu C, David A, Woolf AS, Cuckow PM, Fry CH (2003) Neurotransmission and viscoelasticity in the ovine fetal bladder after in utero bladder outflow obstruction. Am J Physiol Regul Integr Comp Physiol 284:R1296–R1305
Thornbury KD, Hollywood MA, McHale NG (1992) Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol 451:133–144
Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT (2005) Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289:F604–F610
Toji S, Watanabe T, Miyagawa I (2008) Effects of long-term estrogen treatment on micturition behavior and the sensory neurons of the urinary bladder in old female rats. Urol Int 81:462–467
Tomlinson DR, Yusof AP (1983) Autonomic neuropathy in the alloxan-diabetic rat. J Auton Pharmacol 3:257–263
Tong YC, Hung Y-C, Cheng JT (1997) Evidence of P2Y-purinoceptor mediated bladder neck smooth muscle post-contractile relaxation in the male mini-pig. Neurosci Lett 225:181–184
Tong Y-C, Hung Y-C, Lin JSN, Hsu C-T, Cheng J-T (1995) Effects of pregnancy and progesterone on autonomic function in the rat urinary bladder. Pharmacology 50:192–200
Tong YC, Hung YC, Shinozuka K, Kunitomo M, Cheng JT (1997) Evidence of adenosine 5′-triphosphate release from nerve and P2X-purinoceptor mediated contraction during electrical stimulation of rat urinary bladder smooth muscle. J Urol 158:1973–1977
Tran LV, Somogyi GT, de Groat WC (1994) Inhibitory effect of neuropeptide Y on adrenergic and cholinergic transmission in rat urinary bladder and urethra. Am J Physiol 266:R1411–R1417
Tsai MH, Kamm KE, Stull JT (2012) Signalling to contractile proteins by muscarinic and purinergic pathways in neurally stimulated bladder smooth muscle. J Physiol 590:5107–5121
Tugay M, Yildiz F, Utkan T, Gacar N, Ulak G, Erden F (2003) Age-related smooth muscle reactivity changes in the rat bladder: an in vitro study. Pharmacol Res 48:329–334
Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F (1996) Urinary incontinence in women of reproductive age. Gynecol Obstet Invest 41:132–134
Udoh FV (1995) Effects of leaf and root extracts of Nauclea latifolia on purinergic neurotransmission in the rat bladder. Phytother Res 9:239–243
Ursillo RC (1961) Investigation of certain aspects of atropine-resistant nerve effects. J Pharmacol Exp Ther 131:231–236
Usune S, Katsuragi T, Furukawa T (1996) Effects of PPADS and suramin on contractions and cytoplasmic Ca2+ changes evoked by AP4A, ATP and α, β-methylene ATP in guinea-pig urinary bladder. Br J Pharmacol 117:698–702
Utkan T, Erden F, Yildiz F, Özdemirci S, Ulak G, Gacar MN (2001) Chronic ethanol consumption impairs adrenoceptor- and purinoceptor-mediated relaxations of isolated rat detrusor smooth muscle. BJU Int 88:278–283
Uvelius B (1986) Detrusor smooth muscle in rats with alloxan-induced diabetes. J Urol 136:949–952
Uvelius B, Gabella G (1995) Intramural neurones appear in the urinary bladder wall following excision of the pelvic ganglion in the rat. Neuroreport 6:2213–2216
Uvin P, Boudes M, Menigoz A, Franken J, Pinto S, Gevaert T, Verplaetse R, Tytgat J, Vennekens R, Voets T, De Ridder D (2013) Chronic administration of anticholinergics in rats induces a shift from muscarinic to purinergic transmission in the bladder wall. Eur Urol 64:502–510
Vaidyanathan S, Rao MS, Mapa MK, Rao K, Sharma PL, Chary KS (1982) Detrusor supersensitivity to 15(S),15-methyl prostaglandin F2α in chronic neurogenic vesical dysfunction. Indian J Med Res 75:839–845
Vale JA, Liu K, Whitfield HN, Trott KR (1994) Post-irradiation bladder dysfunction: muscle strip findings. Urol Res 22:51–55
Valera S, Hussy N, Evans RJ, Adani N, North RA, Surprenant A, Buell G (1994) A new class of ligand-gated ion channel defined by P2X receptor for extra-cellular ATP. Nature 371:516–519
Valera S, Talabot F, Evans RJ, Gos A, Antonarakis SE, Morris MA, Buell GN (1995) Characterization and chromosomal localization of a human P2X receptor from the urinary bladder. Receptors Channels 3:283–289
Van Nassauw L, Costagliola A, Van Op den bosch J, Cecio A, Vanderwinden J-M, Burnstock G, Timmermans J-P (2006) Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci: Basic Clin 126–127:299–306
Vaught JD, Kropp BP, Sawyer BD, Rippy MK, Badylak SF, Shannon HE, Thor KB (1996) Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: functional innervation and receptor expression. J Urol 155:374–378
Velasco C, Guarneri L, Leonardi A, Testa R (2003) Effects of intravenous and infravesical administration of suramin, terazosin and BMY 7378 on bladder instability in conscious rats with bladder outlet obstruction. BJU Int 92:131–136
Veselá R, Aronsson P, Andersson M, Wsol V, Tobin G (2012) The potential of non-adrenergic, non-cholinergic targets in the treatment of interstitial cystitis/painful bladder syndrome. J Physiol Pharmacol 63:209–216
Veselá R, Aronsson P, Tobin G (2011) Functional and morphological examinations of P1A1 purinoceptors in the normal and inflamed urinary bladder of the rat. Auton Neurosci 159:26–31
Veselá R, Asklund H, Aronsson P, Johnsson M, Wsol V, Andersson M, Tobin G (2012) Coupled nitric oxide and autonomic receptor functional responses in the normal and inflamed urinary bladder of the rat. Physiol Res 61:371–380
Vial C, Evans RJ (2000) P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131:1489–1495
Vizzard MA, Erdman SL, de Groat WC (1996) Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol 370:191–202
Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford APDW, Burnstock G (2001) P2X3 knockout mice reveal a major sensory role for urothelially released ATP. J Neurosci 21:5670–5677
von Kügelgen I (1994) Purinoceptors modulating the release of noradrenaline. J Auton Pharmacol 14:11–12
von Kügelgen I, Schöffel E, Starke K (1989) Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuro-effector transmission in the mouse isolated vas deferens. Naunyn Schmiedebergs Arch Pharmacol 340:522–532
Wada Y, Yoshida M, Kitani K, Kikukawa H, Ichinose A, Takahashi W, Gotoh S, Inadome A, Machida J, Ueda S (1995) Comparison of the effects of various anticholinergic drugs on human isolated urinary bladder. Arch Int Pharmacodyn Ther 330:76–89
Walczak JS, Price TJ, Cervero F (2009) Cannabinoid CB1 receptors are expressed in the mouse urinary bladder and their activation modulates afferent bladder activity. Neuroscience 159:1154–1163
Walsh CA, Cheng Y, Mansfield KJ, Parkin K, Mukerjee C, Moore KH (2013) Decreased intravesical adenosine triphosphate in patients with refractory detrusor overactivity and bacteriuria. J Urol 189:1383–1387
Wammack R, Weihe E, Dienes HP, Hohenfeller R (1995) Die neurogene blase in vitro. Akt Urol 26:16–18
Wang Z, Cristofaro V, Cheng Z, Xiao X, Ge R, Sullivan M, White M, Olumi A (2013) Urothelium-released ATP contributes to bladder dysfunction in type 2 diabetes. J Urol 189:e116
Wang Y, Fang Q, Lu Y, Song B, Li W, Li L (2010) Effects of mechanical stretch on interstitial cells of Cajal in guinea pig bladder. J Surg Res 164:e213–e219
Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G (2005) ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115:2412–2422
Waterman SA (1996) Multiple subtypes of voltage-gated calcium channel mediate transmitter release from parasympathetic neurons in the mouse bladder. J Neurosci 16:4155–4161
Wazir R, Luo DY, Tian Y, Yue X, Li H, Wang KJ (2013) The purinergic component of human bladder smooth muscle cells' proliferation and contraction under physiological stretch. Biochem Biophys Res Commun 437:256–260
Webb HE, Chew-Lim M, Jagelman S, Oaten SW, Pathak A, Suckling AJ, MacKenzie A (1978) Semliki forest virus infections in mice as a model for studying acute and chronic CNS virus infections in man. In: Clifford Rose F (ed) Clinical neuroimmunology. Blackwell, Oxford, pp 369–390
Weetman DF, Turner N (1977) The effects of ATP-receptor blocking agents on the response to the guinea-pig isolated bladder preparation to hyoscine-resistant nerve stimulation. Arch Int Pharmacodyn Ther 228:10–14
Welford LA, Cusack NJ, Hourani SMO (1987) The structure–activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol 141:123–130
Werkström V, Andersson KE (2005) ATP- and adenosine-induced relaxation of the smooth muscle of the pig urethra. BJU Int 96:1386–1391
Werkström V, Persson K, Andersson K-E (1997) NANC transmitters in the female pig urethra — localization and modulation of release via α2-adrenoceptors and potassium channels. Br J Pharmacol 121:1605–1612
Westfall DP, Fedan JS, Colby J, Hogaboom GK, O'Donnell JP (1983) Evidence for a contribution by purines to the neurogenic response of the guinea-pig urinary bladder. Eur J Pharmacol 87:415–422
Westfall TD, Kennedy C, Sneddon P (1997) The ecto-ATPase inhibitor ARL 67156 enhances parasympathetic neurotransmission in the guinea-pig urinary bladder. Eur J Pharmacol 329:169–173
Wiklund NP, Gustafsson LE (1986) Neuromodulation by adenine nucleotides, as indicated by experiments with inhibitors of nucleotide inactivation. Acta Physiol Scand 126:217–223
Wiklund NP, Gustafsson LE (1988) Indications for P2-purinoceptor subtypes in guinea pig smooth muscle. Eur J Pharmacol 148:361–370
Wu C, Bayliss M, Newgreen D, Mundy AR, Fry CH (1999) A comparison of the mode of action of ATP and carbachol on isolated human detrusor smooth muscle. J Urol 162:1840v–1847v
Wu C, Gui GP, Fry CH (2011) Intracellular Ca2+ regulation and electrophysiolgical properties of bladder urothelium subjected to stretch and exogenous agonists. Cell Calcium 49:395–399
Wu C, Sui GP, Fry CH (2004) Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559:231–243
Wu C, Wallis WRJ, Fry CH (1995) Purinergic activation induces a transient rise of intracellular Ca2+ in isolated human detrusor myocytes. J Physiol 489:136P–137P
Wuest M, Morgenstern K, Graf EM, Braeter M, Hakenberg OW, Wirth MP, Ravens U (2005) Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol 173:2182–2189
Wyndaele JJ, De Wachter S (2003) The basics behind bladder pain: a review of data on lower urinary tract sensations. Int J Urol 10:S49–S55
Xiao H, Si LY, Liu W, Li N, Meng G, Yang N, Chen X, Zhou YG, Shen HY (2013) The effects of adenosine A2A receptor knockout on renal interstitial fibrosis in a mouse model of unilateral ureteral obstruction. Acta Histochem 115:315–319
Yamada S, Yoshida A, Kageyama A, Mori F, Ito Y (2009) Muscarinic and purinergic receptors in the rat bladder are altered by chemically-induced cystitis. Neurourol Urodyn 28:825–826
Yang SJ, An JY, Shim JO, Park CH, Huh IH, Sohn UD (2000) The mechanism of contraction by 2-chloroadenosine in cat detrusor muscle cells. J Urol 163:652–658
Yeh CH, Chiang HS, Chien CT (2010) Hyaluronic acid ameliorates bladder hyperactivity via the inhibition of H2O2-enhanced purinergic and muscarinic signaling in the rat. Neurourol Urodyn 29:765–770
Yiangou Y, Facer P, Ford A, Brady C, Wiseman O, Fowler CJ, Anand P (2001) Capsaicin receptor VR1 and ATP-gated ion channel P2X3 in human urinary bladder. BJU Int 87:774–779
Yokota T, Yamaguchi O (1996) Changes in cholinergic and purinergic neurotransmission in pathologic bladder of chronic spinal rabbit. J Urol 156:1862–1866
Yokoyama O, Tanaka I, Kusukawa N, Yamauchi H, Ito H, Aoki Y, Oyama N, Miwa Y, Akino H (2011) Antimuscarinics suppress adenosine triphosphate and prostaglandin E2 release from urothelium with potential improvement in detrusor overactivity in rats with cerebral infarction. J Urol 185:2392–2397
Yoshida A, Kageyama A, Fujino T, Nozawa Y, Yamada S (2010) Loss of muscarinic and purinergic receptors in urinary bladder of rats with hydrochloric acid-induced cystitis. Urology 76:1017–12
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23
Yoshida M, Nagata T, Masunaga K, Inadome A, Miyamoto Y, Haba T, Kudoh J (2011) Contribution of non-neuronal adenosine triphosphate release from bladder mucosa to detrusor overactivity in hydrochloric acid (HCL)-induced cystitis rats. Eur Urol Suppl 10:257–258
Yoshimura N (1999) Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol 57:583–606
Yoshimura N, de Groat WC (1997) Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol 503:269–276
Yoshimura N, de Groat WC (1999) Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19:4644–4653
Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, Tyagi P (2008) Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol 377:437–448
Young JS, Matharu R, Carew MA, Fry CH (2012) Inhibition of stretching-evoked ATP release from bladder mucosa by anticholinergic agents. BJU Int 110:E397–E401
Young JS, Meng E, Cunnane TC, Brain KL (2008) Spontaneous purinergic neurotransmission in the mouse urinary bladder. J Physiol 586:5743–5755
Yu Y, de Groat WC (2008) Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder–pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol 294:F1146–F1156
Yu Y, de Groat WC (2013) Nitric oxide modulates bladder afferent nerve activity in the in vitro urinary bladder–pelvic nerve preparation from rats with cyclophosphamide induced cystitis. Brain Res 1490:83–94
Yu W, Hill WG (2011) Defining protein expression in the urothelium: a problem of more than transitional interest. Am J Physiol Renal Physiol 301:F932–F942
Yu W, Robson SC, Hill WG (2011) Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One 6:e18704
Yu W, Sun X, Robson SC, Hill WG (2013) Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway. FASEB J 27:1895–1903
Yu W, Zeidel ML, Hill WG (2012) Cellular expression profile for interstitial cells of Cajal in bladder — a cell often misidentified as myocyte or myofibroblast. PLoS One 7:e48897
Zagorodnyuk VP, Costa M, Brookes SJ (2006) Major classes of sensory neurons to the urinary bladder. Auton Neurosci 126–127:390–397
Zar MA, Iravani MM, Luheshi GN (1990) Effect of nifedipine on the contractile responses of the isolated rat bladder. J Urol 143:835–839
Zderic SA, Plzak JE, Duckett JW, Snyder HM III, Wein AJ, Levin RM (1990) Effect of pregnancy on rabbit urinary bladder physiology: 1. Effects of extracellular calcium. Pharmacology 41:124–129
Zhang SX, Kobayashi T, Okada T, Garcia del Saz E, Seguchi H (1991) Alkaline phosphatase, 5′-nucleotidase and magnesium-dependent adenosine triphosphatase activities in the transitional epithelium of the rat urinary bladder. Histol Histopathol 6:309–315
Zhang S, Lv JW, Yang P, Yu Q, Pang J, Wang Z, Guo H, Liu S, Hu J, Li J, Leng J, Huang Y, Ye Z, Wang CY (2012) Loss of dicer exacerbates cyclophosphamide-induced bladder overactivity by enhancing purinergic signaling. Am J Pathol 181:937–946
Zhao M, Bo X, Neely CF, Burnstock G (1996) Characterization and autoradiographic localization of [3H]α, β-methylene ATP binding sites in cat urinary bladder. Gen Pharmacol 27:509–512
Zhao Y, Wein AJ, Levin RM (1993) Role of calcium in mediating the biphasic contraction of the rabbit urinary bladder. Gen Pharmacol 24:727–731
Zhong Y, Banning AS, Cockayne DA, Ford APDW, Burnstock G, McMahon SB (2003) Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience 120:667–675
Zhong Y, Dunn PM, Burnstock G (2000) Guinea-pig sympathetic neurons express varying proportions of two distinct P2X receptors. J Physiol 523:391–402
Zhong Y, Dunn PM, Xiang Z, Bo X, Burnstock G (1998) Pharmacological and molecular characterisation of P2X purinoceptors in rat pelvic ganglion neurons. Br J Pharmacol 125:771–781
Ziganshin AU, Hoyle CHV, Bo X, Lambrecht G, Mutschler E, Bäumert HG, Burnstock G (1993) PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder. Br J Pharmacol 110:1491–1495
Ziganshin AU, Hoyle CHV, Ziganshina LE, Burnstock G (1994) Effects of cyclopiazonic acid on contractility and ecto-ATPase activity in guinea-pig urinary bladder and vas deferens. Br J Pharmacol 113:669–674
Ziganshin AU, Ralevic V, Burnstock G (1995) Contractility of urinary bladder and vas deferens after sensory denervation by capsaicin treatment of newborn rats. Br J Pharmacol 114:166–170
Ziganshin AU, Ziganshina LE, Hoyle CHV, Burnstock G (1995) Effects of divalent cations and La3+ on contractility and ecto-ATPase activity in the guinea-pig urinary bladder. Br J Pharmacol 114:632–639
Zimmermann H (1978) Turnover of adenine nucleotides in cholinergic synaptic vesicles of the Torpedo electric organ. Neuroscience 3:827–836
Zimmermann H (2006) Ectonucleotidases in the nervous system. Novartis Foundation Symposium 276 Purinergic signalling in neuron–glial interactions. John Wiley & Sons, Ltd, Chichester, pp 113–128
Zinck ND, Downie JW (2008) IB4 afferent sprouting contributes to bladder dysfunction in spinal rats. Exp Neurol 213:293–302
Zoubek J, Somogyi GT, de Groat WC (1993) A comparison of inhibitory effects of neuropeptide Y on rat urinary bladder, urethra, and vas deferens. Am J Physiol 265:R537–R543
Acknowledgments
Anthony Ford made particularly helpful suggestions that improved the first draft of this review. The author is very grateful to Dr Gillian E. Knight for her invaluable assistance in the preparation of this review article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burnstock, G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signalling 10, 103–155 (2014). https://doi.org/10.1007/s11302-013-9395-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-013-9395-y