Abstract
Purpose
In this study, we compared firefly luciferase (Fluc) reporter gene and superparamagnetic iron oxide (Feridex) as cell markers for longitudinal monitoring of cardiomyoblast graft survival using optical bioluminescence imaging (BLI) and magnetic resonance imaging (MRI), respectively.
Procedures
Rats (n = 31) underwent an intramyocardial injection of cardiomyoblasts (2 × 106) labeled with Fluc, Feridex, or no marker (control) or an injection of Feridex alone (75 μg). Afterward, rats were serially imaged with BLI or MRI and killed at different time points for histological analysis.
Results
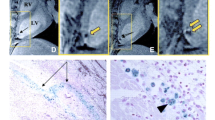
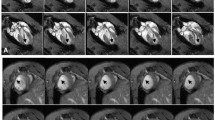
BLI revealed a drastically different cell survival kinetics (half-life = 2.65 days over 6 days) than that revealed by MRI (half-life = 16.8 days over 80 days). Injection of Feridex alone led to prolonged tissue retention of Feridex (≥16 days) and persistent MR signal (≥42 days).
Conclusions
Fluc BLI reporter gene imaging is a more accurate gauge of transplanted cell survival as compared to MRI of Feridex-labeled cells.






Similar content being viewed by others
References
Reffelmann T, Kloner RA (2003) Cellular cardiomyoplasty–cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure? Cardiovasc Res 58:358–368
Rosenzweig A (2006) Cardiac cell therapy—mixed results from mixed cells. N Engl J Med 355:1274–1277
Janssens S et al (2006) Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367:113–121
Frangioni JV, Hajjar RJ (2004) In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation 110:3378–3383
Bengel FM, Schachinger V, Dimmeler S (2005) Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging 32(Suppl 2):S404–S416
Zhou R, Acton PD, Ferrari VA (2006) Imaging stem cells implanted in infarcted myocardium. J Am Coll Cardiol 48:2094–2106
Wu JC et al (2003) Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation 108:1302–1305
Zhou R et al (2005) In vivo detection of stem cells grafted in infarcted rat myocardium. J Nucl Med 46:816–822
Kraitchman DL et al (2003) In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation 107:2290–2293
Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET (2005) A new transgene reporter for in vivo magnetic resonance imaging. Nat Med 11:450–454
Hofmann M et al (2005) Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 111:2198–2202
Ray P et al (2001) Monitoring gene therapy with reporter gene imaging. Semin Nucl Med 31:312–320
Chen IY et al (2004) Micro-positron emission tomography imaging of cardiac gene expression in rats using bicistronic adenoviral vector-mediated gene delivery. Circulation 109:1415–1420
Gheysens O et al (2006) Noninvasive evaluation of immunosuppressive drug efficacy on acute donor cell survival. Mol Imaging Biol 8:163–170
Matuszewski L et al (2005) Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology 235:155–161
Arbab AS et al (2003) Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology 229:838–846
Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW (2004) Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 17:513–517
Wu JC et al (2006) Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics 25:29–38
Wu JC et al (2006) Proteomic analysis of reporter genes for molecular imaging of transplanted embryonic stem cells. Proteomics 6:6234–6249
Cao F et al (2006) In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 113:1005–1014
Johansen J et al (2002) Evaluation of Tet-on system to avoid transgene down-regulation in ex vivo gene transfer to the CNS. Gene Ther 9:1291–1301
Derouazi M et al (2006) Genetic characterization of CHO production host DG44 and derivative recombinant cell lines. Biochem Biophys Res Commun 340:1069–1077
Olivares EC, Hollis RP, Chalberg TW, Meuse L, Kay MA, Calos MP (2002) Site-specific genomic integration produces therapeutic Factor IX levels in mice. Nat Biotechnol 20:1124–1128
Krishnan M et al (2006) Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. Faseb J 20:106–108
Nakamura Y, Yasuda T, Weisel RD, Li RK (2006) Enhanced cell transplantation: preventing apoptosis increases cell survival and ventricular function. Am J Physiol Heart Circ Physiol 291:H939–H947
Muller-Ehmsen J et al (2002) Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol 34:107–116
Yau TM, Kim C, Li G, Zhang Y, Weisel RD, Li RK (2005) Maximizing ventricular function with multimodal cell-based gene therapy. Circulation 112:I123–I128
Himes N et al (2004) In vivo MRI of embryonic stem cells in a mouse model of myocardial infarction. Magn Reson Med 52:1214–1219
Naylor LH (1999) Reporter gene technology: the future looks bright. Biochem Pharmacol 58:749–757
Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17:545–580
Bos C et al (2004) In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology 233:781–789
Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM (2005) Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med 53:999–1005
Foltz WD, Cunningham CH, Mutsaers AJ, Conolly SM, Stewart DJ, Dick AJ (2006) Positive-contrast imaging in the rabbit hind-limb of transplanted cells bearing endocytosed superparamagnetic beads. J Cardiovasc Magn Reson 8:817–823
Acknowledgments
This work was supported in part by NHLBI 5R01HL078632 (S.S.G.), NCI ICMIC P50 CA114747 (S.S.G.), NCI SAIRP (S.S.G.), American Heart Association Pre-doctoral Fellowship (I.Y.C.), Stanford Bio-X Graduate Student Fellowship (I.Y.C), Swiss Foundation of Medical-Biological Grants (JKW), Novartis Research Foundation (JKW), Swiss Society of Radiology (JKW), and AHA Beginning Grant in Aid (J.C.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, I.Y., Greve, J.M., Gheysens, O. et al. Comparison of Optical Bioluminescence Reporter Gene and Superparamagnetic Iron Oxide MR Contrast Agent as Cell Markers for Noninvasive Imaging of Cardiac Cell Transplantation. Mol Imaging Biol 11, 178–187 (2009). https://doi.org/10.1007/s11307-008-0182-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0182-z