Abstract
Purpose
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) measured the early vascular changes after administration of TRA-8, bevacizumab, or TRA-8 combined with bevacizumab in breast tumor xenografts.
Procedures
Groups 1–4 of nude mice bearing human breast carcinoma were injected with phosphate-buffered saline, TRA-8, bevacizumab, and TRA-8 + bevacizumab on day 0, respectively. DCE-MRI was performed on days 0, 1, 2, and 3, and thereafter tumors were collected for terminal deoxynucleotidyl transferase-mediated dUT nick end labeling and CD31 staining.
Results
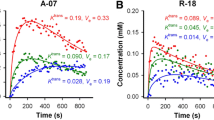
DCE-MRI measured a significant K trans change within 3 days after TRA-8 therapy that correlated with tumor growth arrest, which was not shown with statistical significance by histopathology at these early time points posttreatment. The K trans changes followed quadratic polynomial curves.
Conclusion
DCE-MRI detected significantly lower K trans levels in breast tumor xenografts following TRA-8 monotherapy or combined therapy with bevacizumab.






Similar content being viewed by others
References
Sheridan JP, Marsters SA, Pitti RM et al (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277:818–821
Hylander BL, Pitoniak R, Penetrante RB et al (2005) The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med 3:22
Jo M, Kim TH, Seol DW et al (2000) Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 6:564–567
Emery JG, McDonnell P, Burke MB et al (1998) Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 273:14363–14367
Ichikawa K, Liu W, Zhao L et al (2001) Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 7:954–960
Forero-Torres A, Shah J, Wood T et al (2009) Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother Radiopharm 25:13–19
Buchsbaum DJ, Zhou T, Grizzle WE et al (2003) Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res 9:3731–3741
Evelhoch JL, Gillies RJ, Karczmar GS et al (2000) Applications of magnetic resonance in model systems: cancer therapeutics. Neoplasia 2:152–165
Cheung YC, Chen SC, Su MY et al (2003) Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat 78:51–58
Martincich L, Montemurro F, De Rosa G et al (2004) Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat 83:67–76
Wedam SB, Low JA, Yang SX et al (2006) Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 24:769-777
Liu G, Rugo HS, Wilding G et al (2005) Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol 23:5464–5473
Spinosa DJ, Kaufmann JA, Hartwell GD (2002) Gadolinium chelates in angiography and interventional radiology: a useful alternative to iodinated contrast media for angiography. Radiology 223:319–325
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Kim YR, Rebro KJ, Schmainda KM (2002) Water exchange and inflow affect the accuracy of T1-GRE blood volume measurements: implications for the evaluation of tumor angiogenesis. Magn Reson Med 47:1110–1120
Yankeelov TE, Luci JJ, Lepage M et al (2005) Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn Reson Imaging 23:519–529
Yankeelov TE, Cron GO, Addison CL et al (2007) Comparison of a reference region model with direct measurement of an AIF in the analysis of DCE-MRI data. Magn Reson Med 57:353–361
Kim H, Morgan DE, Zeng H et al (2008) Breast tumor xenografts: diffusion-weighted MR imaging to assess early therapy with novel apoptosis-inducing anti-DR5 antibody. Radiology 248:844–851
Sternberg SR (1983) Biomedical image processing. IEEE Computer 16:22–34
Hertzog C, Rovine M (1985) Repeated-measures analysis of variance in developmental research: selected issues. Child Dev 56:787–809
Maxwell SE (1980) Pairwise multiple comparisons in repeated measures designs. J Educ Behav Stat 5:269–287
Neter J, Kutner MH, Nachtsheim JC, Wasserman W (1996) Applied linear statistical models. McGraw-Hill, Columbus
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. Freeman, New York
Rodgers JL, Nicewander WA (1988) Thirteen ways to look at the correlation coefficient. Am Stat 42:59–66
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Wosik K, Biernacki K, Khouzam MP, Prat A (2007) Death receptor expression and function at the human blood brain barrier. J Neurol Sci 259:53–60
Su MY, Baik HM, Yu HJ, Chen JH, Mehta RS, Nalcioglu O (2006) Comparison of choline and pharmacokinetic parameters in breast cancer measured by MR spectroscopic imaging and dynamic contrast enhanced MRI. Technol Cancer Res Treat 5:401–410
Morse DL, Raghunand N, Sadarangani P et al (2007) Response of choline metabolites to docetaxel therapy is quantified in vivo by localized (31)P MRS of human breast cancer xenografts and in vitro by high-resolution (31)P NMR spectroscopy of cell extracts. Magn Reson Med 58:270–280
Jacobs MA, Barker PB, Bottomley PA, Bhujwalla Z, Bluemke DA (2004) Proton magnetic resonance spectroscopic imaging of human breast cancer: a preliminary study. J Magn Reson Imaging 19:68–75
Momiyama N, Ishikawa T, Ichikawa Y, Shimada H, Katayama A, Ozawa Y (2007) [Early prediction of response to primary chemotherapy by sequential FDG -PET in patients with advanced breast cancer]. Nippon Rinsho 65 Suppl 6:385–388
Kiessling F, Farhan N, Lichy MP et al (2004) Dynamic contrast-enhanced magnetic resonance imaging rapidly indicates vessel regression in human squamous cell carcinomas grown in nude mice caused by VEGF receptor 2 blockade with DC101. Neoplasia 6:213–223
Wilmes LJ, Pallavicini MG, Fleming LM et al (2007) AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging 25:319–327
Grubbs CJ, Hill DL, Bland KI et al (2003) 9cUAB30, an RXR specific retinoid, and/or tamoxifen in the prevention of methylnitrosourea-induced mammary cancers. Cancer Lett 201:17–24
Fiebig HH, Maier A, Burger AM (2004) Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer 40:802–820
Grobner T, Prischl FC (2007) Gadolinium and nephrogenic systemic fibrosis. Kidney Int 72:260–264
McLachlan SJ, Eaton S, De Simone DN (1992) Pharmacokinetic behavior of gadoteridol injection. Invest Radiol 27 Suppl 1:S12–S15
Kim H, Chaudhuri TR, Buchsbaum DJ, Wang D, Zinn KR (2007) High-resolution single-photon emission computed tomography and X-ray computed tomography imaging of Tc-99 m-labeled anti-DR5 antibody in breast tumor xenografts. Mol Cancer Ther 6:866–875
Niermann KJ, Fleischer AC, Huamani J et al (2007) Measuring tumor perfusion in control and treated murine tumors: correlation of microbubble contrast-enhanced sonography to dynamic contrast-enhanced magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. J Ultrasound Med 26:749–756
Carano RA, Ross AL, Ross J et al (2004) Quantification of tumor tissue populations by multispectral analysis. Magn Reson Med 51:542–551
Acknowledgements
Financial support and TRA-8 were obtained from Daiichi Sankyo. Support was also provided by an HSF-GEF Scholar Award, Research Initiative Pilot Award from the Department of Radiology at UAB, NIH grants 5P50CA89019, P20CA101955-05, and 5P30CA013148, and Susan G. Komen Breast Cancer Foundation BCTR0600484 and KG090969.
Conflict of Interest
Donald Buchsbaum and UAB have intellectual property interest related to TRA-8.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Mouse Port Implantation and Maintenance
Each mouse was anesthetized with an intraperitoneal injection of sodium pentobarbital (60–65 mg/kg BW, Nembutal sodium solution, Abbott Laboratories, North Chicago, IL, USA) in 0.2 ml of saline and placed in supine position on the operative field. A 0.7∼1.0-cm incision in an area of the mouse back was made for implanting the mouse port. A subcutaneous pocket was made by insertion of hemostats (micro-mosquito hemostat, Fine Science Tools Inc., Foster City, CA, USA), and then the port was implanted into the pocket. Another skin incision (0.5 cm) was made in the neck area to expose a jugular vein, and a canal was created under the skin between two incisions using a straight forceps. The catheter connected to the port was held and dragged through the canal and was introduced into the jugular vein. The vein was isolated and secured with two 7-0 sutures. After surgery, each mouse was injected intramuscularly with 2 mg/kg BW of buprenorphine hydrochloride (Buprenex, Hospira Inc., Lake Forest, IL, USA) in 0.2 ml of saline for analgesia. The cage containing the mice was then placed on a SoftHeat Heating Pad (Kaz Inc., Southborough, MA, USA) for about 1 h during recovery from surgery. The port was rinsed with heparin (8.6 U/ml) in 0.1 ml of PBS (pH 7.4) every 24 h to prevent blood coagulation inside of the catheter. The lumen of the port had 0.05-ml dead volume, so the heparin solution was mixed with gadoteridol (0.0267 mmol/ml) to fill the dead volume with gadoteridol and avoid dilution.
Appendix B: Tumor Tissue Staining
Each tumor was sliced into two pieces and then immersed into 10% neutral-buffered formalin overnight at room temperature. Tissue sections of 5-µm thickness were cut on an Accu-Cut SRM microtome (Sakura, Tokyo, Japan). Sections of paraffin-embedded tissue were mounted on Bond-Rite slides from Richard-Allan Scientific (Kalamazoo, MI, USA) and heated at 60°C for 2 h. Paraffin was removed from the sections by three changes of xylene and rehydrated through graded alcohols from absolute to 70% for 5 min each.
Antigen retrieval was performed with high-temperature treatment with 0.5 M Tris buffer at pH 10. H2O2 avidin and biotin solutions and 3% goat serum were used to quench peroxidases, block endogenous biotin, and block nonspecific binding. Rabbit polyclonal antibody to CD31 (Abcam Inc., Cambridge, MA, USA) was diluted 1:200 and applied to the tissue at room temperature for 1 h. The secondary antibody was goat antirabbit (Jackson Immuno Research, West Grove, PA, USA) and the label was avidin–HRP (Signet Pathology Systems, Dedham, MA, USA). After the DAB chromagen (BioGenex, San Ramon, CA, USA) was applied, the tissues were counterstained with hematoxylin and the coverslips mounted with Permount.
The TUNEL assay was performed with a Chemicon International ApopTag Peroxidase In Situ Detection kit (Temecula, CA, USA). The slides were rehydrated as above and pretreated for 1 min in 10 mM glycine at pH 3 with fast cooling after heating in a pressure cooker. The slides were rinsed for a minimum of 2 h with deionized water after quenching with H2O2. The chromagen used was 3-3′diaminobenzidine according to the manufacturer’s protocol (BioGenex, San Ramon, CA, USA). After 7 min, the slides were rinsed with water and lightly counterstained with Mayer’s hematoxylin. The sections were dehydrated through graded alcohols, 70% to absolute ethanol, followed by three xylene rinses for 5 min each. The coverslips were mounted with Permount.
Rights and permissions
About this article
Cite this article
Kim, H., Folks, K.D., Guo, L. et al. DCE-MRI Detects Early Vascular Response in Breast Tumor Xenografts Following Anti-DR5 Therapy. Mol Imaging Biol 13, 94–103 (2011). https://doi.org/10.1007/s11307-010-0320-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0320-2