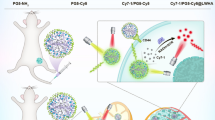
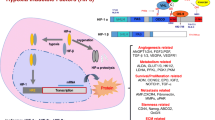
Abstract
Purpose
Hypoxia is commonly observed in regions of primary tumors and metastases, and is associated with resistance to treatment, more aggressive tumor phenotypes and poor prognosis. Reliable and validated imaging biomarkers of hypoxia are needed for pre-clinical studies and clinical use. Expression of cell-surface carbonic anhydrases IX and XII (CAIX and CAXII) in tumor cells has been associated with tumor hypoxia. CAIX and CAXII specific antibodies conjugated to fluorescent dye were evaluated for the non-invasive detection of hypoxia in vivo.
Procedures
Human breast cancer cell lines (MCF10A, DCIS, MCF7, ZR-75.1 and MDA-mb231) were characterized for CAIX and CAXII expression by real-time RT-PCR and immunocytochemistry (ICC) under normoxic and hypoxic conditions. Immunohistochemical (IHC) staining of CAIX, CAXII and the commercially available exogenous hypoxia marker, pimonidazole, was performed using sections of ZR-75.1 and MDA-mb-231 orthotopic breast cancer xenograft tumors from nude mice. In vivo fluorescence imaging of ZR-75.1 tumors in animals housed at varied levels of oxygen was used to quantify the relative uptake of the CAIX and CAXII agents and a commercially available sulfonamide-based agent. Corresponding tumor sections were IHC stained for CAIX, CAXII and pimonidazole.
Results
CAIX mRNA expression was significantly higher (p < 0.05) in hypoxia for all cell lines, which was in agreement with protein expression by ICC. CAXII expression was mixed, with a modest hypoxia-related increase in two cell lines (p < 0.05) and no change in others. Quantified IHC staining of ZR-75.1 and MDA-mb-231 tumor sections showed that CAIX and CAXII expression was elevated in regions with pimonidazole staining, but CAXII levels were lower than CAIX. Tumor uptake of the CAIX targeted agent, and IHC staining of CAIX and pimonidazole in corresponding tumor sections were correlated, and co-registered, and shown to be significantly elevated by level of oxygenation (p < 0.001): hypoxia > normoxia > hyperoxia. However, the CAXII and sulfonamide agents were not significantly correlated with hypoxia.
Conclusion
These studies suggest that the fluorescently labeled CAIX-specific agent is a more robust indicator of hypoxia in vivo compared to the CAXII-specific agent or the agent specific to the CA active site.






Similar content being viewed by others
Abbreviations
- CAIX:
-
Carbonic anhydrase IX
- CAXII:
-
Carbonic anhydrase XII
References
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Cairns RA, Papandreou I, Sutphin PD, Denko NC (2007) Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci U S A 104:9445–9450
Wojtkowiak JW, Cornnell HC, Matsumoto S et al (2015) Pyruvate sensitizes pancreatic tumors to hypoxia-activated prodrug TH-302. Cancer Metabol 3:2
Wouters A, Pauwels B, Lardon F, Vermorken JB (2007) Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist 12:690–712
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2:38–47
Kaanders JH, Wijffels KI, Marres HA et al (2002) Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res 62:7066–7074
Sun JD, Liu Q, Wang J et al (2012) Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res 18:758–770
Evans SM, Koch CJ (2003) Prognostic significance of tumor oxygenation in humans. Cancer Lett 195:1–16
Sun X, Niu G, Chan N et al (2011) Tumor hypoxia imaging. Mol Imaging Biol 13:399–410
Gatenby RA, Kessler HB, Rosenblum JS et al (1988) Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 14:831–838
Nozue M, Lee I, Yuan F et al (1997) Interlaboratory variation in oxygen tension measurement by Eppendorf “Histograph” and comparison with hypoxic marker. J Surg Oncol 66:30–38
Jenkins WT, Evans SM, Koch CJ (2000) Hypoxia and necrosis in rat 9L glioma and Morris 7777 hepatoma tumors: comparative measurements using EF5 binding and the Eppendorf needle electrode. Int J Radiat Oncol Biol Phys 46:1005–1017
He F, Deng X, Wen B et al (2008) Noninvasive molecular imaging of hypoxia in human xenografts: comparing hypoxia-induced gene expression with endogenous and exogenous hypoxia markers. Cancer Res 68:8597–8606
Bussink J, Kaanders JH, van der Kogel AJ (2003) Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol 67:3–15
Olive PL, Aquino-Parsons C, MacPhail SH et al (2001) Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res 61:8924–8929
Wykoff CC, Beasley NJ, Watson PH et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60:7075–7083
Koch CJ, Evans SM, Lord EM (1995) Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)a cet amide] : analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer 72:869–874
Raleigh JA, Calkins-Adams DP, Rinker LH et al (1998) Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res 58:3765–3768
Mees G, Dierckx R, Vangestel C, Van de Wiele C (2009) Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging 36:1674–1686
Cook GJ (2003) Oncological molecular imaging: nuclear medicine techniques. Br J Radiol 76(Spec No 2):S152–S158
Pastorekova S, Zatovicova M, Pastorek J (2008) Cancer-associated carbonic anhydrases and their inhibition. Curr Pharm Des 14:685–698
Chi JT, Wang Z, Nuyten DS et al (2006) Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med 3:e47
Winum JY, Scozzafava A, Montero JL, Supuran CT (2009) Inhibition of carbonic anhydrase IX: a new strategy against cancer. Anti cancer Agents Med Chem 9:693–702
Zatovicova M, Jelenska L, Hulikova A et al (2010) Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des 16:3255–3263
Supuran CT, Scozzafava A (2007) Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 15:4336–4350
Ivanov S, Liao SY, Ivanova A et al (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158:905–919
Tafreshi NK, Bui MM, Bishop K et al (2012) Noninvasive detection of breast cancer lymph node metastasis using carbonic anhydrases IX and XII targeted imaging probes. Clin Cancer Res 18:207–219
Watson PH, Chia SK, Wykoff CC et al (2003) Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer 88:1065–1070
Ivanov SV, Kuzmin I, Wei MH et al (1998) Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci U S A 95:12596–12601
Pastorekova S, Parkkila S, Zavada J (2006) Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 42:167–216
Bahnson EE, Murrey DA, Mojzisik CM et al (2009) PET/CT imaging of clear cell renal cell carcinoma with I labeled chimeric antibody. Ther Adv Urol 1:67–70
Divgi CR, Pandit-Taskar N, Jungbluth AA et al (2007) Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 8:304–310
Dubois L, Lieuwes NG, Maresca A et al (2009) Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol 92:423–428
Barathova M, Takacova M, Holotnakova T et al (2008) Alternative splicing variant of the hypoxia marker carbonic anhydrase IX expressed independently of hypoxia and tumour phenotype. Br J Cancer 98:129–136
Morse DL, Carroll D, Weberg L et al (2005) Determining suitable internal standards for mRNA quantification of increasing cancer progression in human breast cells by real-time reverse transcriptase polymerase chain reaction. Anal Biochem 342:69–77
Kaanders JH, Pop LA, Marres HA et al (1998) Accelerated radiotherapy with carbogen and nicotinamide (ARCON) for laryngeal cancer. Radiother Oncol 48:115–122
Kumar AT, Raymond SB, Dunn AK et al (2008) A time domain fluorescence tomography system for small animal imaging. IEEE Trans Med Imaging 27:1152–1163
Kim J (2013) The computation of bivariate normal and t probabilities, with application to comparisons of three normal means. Comput Stat Data Anal 58:177–186
Tafreshi NK, Enkemann SA, Bui MM et al (2011) A mammaglobin—a targeting agent for noninvasive detection of breast cancer metastasis in lymph nodes. Cancer Res 71:1050–1059
Varia MA, Calkins-Adams DP, Rinker LH et al (1998) Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol Oncol 71:270–277
Chiche J, Ilc K, Laferriere J et al (2009) Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 69:358–368
Beasley NJ, Wykoff CC, Watson PH et al (2001) Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res 61:5262–5267
Carlin S, Khan N, Ku T et al (2010) Molecular targeting of carbonic anhydrase IX in mice with hypoxic HT29 colorectal tumor xenografts. PLoS One 5:e10857
van Laarhoven HW, Bussink J, Lok J et al (2004) Effects of nicotinamide and carbogen in different murine colon carcinomas: immunohistochemical analysis of vascular architecture and microenvironmental parameters. Int J Radiat Oncol Biol Phys 60:310–321
Onogawa S, Kitadai Y, Tanaka S et al (2004) Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci 95:32–39
Svastova E, Hulikova A, Rafajova M et al (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 577:439–445
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181
Brouwers AH, Buijs WC, Oosterwijk E et al (2003) Targeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with (131)I or (111) In: an intrapatient comparison. Clin Cancer Res 9:3953S–3960S
Acknowledgments
The authors wish to acknowledge the following core facilities at the H. Lee Moffitt Cancer Center & Research Institute for their contributions to this work: Analytic Microscopy, Tissue, Molecular Genomics, Comparative Biomedicine and the Small Animal Imaging Laboratory. This work was supported in part by the Cancer Center Support Grant P30 CA076292 from the National Cancer Institute. NIH Grant Support: NIH/NCI R01CA077575 (RJG).
Conflict of Interest
All authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tafreshi, N.K., Lloyd, M.C., Proemsey, J.B. et al. Evaluation of CAIX and CAXII Expression in Breast Cancer at Varied O2 Levels: CAIX is the Superior Surrogate Imaging Biomarker of Tumor Hypoxia. Mol Imaging Biol 18, 219–231 (2016). https://doi.org/10.1007/s11307-015-0885-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0885-x