Abstract
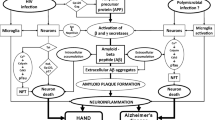
The use of antiretroviral therapy for HIV infection has extended the survival of individuals living with HIV. However, the effects of chronic HIV infection and aging are introducing another facet of HIV complications. HIV therapy can calm the immune system and lower viral replication to undetectable but the virus is still present. In the brain, amyloid beta (Aβ) increases during normal aging but Aβ accumulation appears to accelerate in HIV infection. HIV Tat protein inhibits the major Aβ-degrading enzyme neprilysin with the cysteine-rich domain of Tat being essential for this inhibition. In this minireview, we also include new data that the β chemokine, CCL2/MCP-1, associated with HIV migration to the brain, also causes an increase in Aβ. These findings may explain the continued cognitive dysfunction found in HIV-infected individuals controlled on antiviral therapy.




Similar content being viewed by others
References
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Carson JA, Turner AJ (2002) Beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J Neurochem 81:1–8. doi:10.1046/j.1471-4159.2002.00855.x
Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11:1421–1431. doi:10.1097/00002030-199712000-00006
Chesneau V, Vekrellis K, Rosner MR, Selkoe DJ (2000) Purified recombinant insulin-degrading enzyme degrades amyloid beta-protein but does not promote its oligomerization. Biochem J 351(Pt 2):509–516. doi:10.1042/0264-6021:3510509
Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L (1988) Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38:1682–1687
Cummings BJ, Pike CJ, Shankle R, Cotman CW (1996) Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiol Aging 17:921–933. doi:10.1016/S0197-4580(96)00170-4
Daily A, Nath A, Hersh LB (2006) Tat peptides inhibit neprilysin. J Neurovirology 12:153–160. doi:10.1080/13550280600760677
Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol 67:277–287
Esiri MM, Biddolph SC, Morris CS (1998) Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry 65:29–33. doi:10.1136/jnnp.65.1.29
Ferreira ST, Vieira MN, De Felice FG (2007) Soluble protein oligomers as emerging toxins in Alzheimer’s and other amyloid diseases. IUBMB Life 59:332–345. doi:10.1080/15216540701283882
Gelman BB, Schuenke K (2004) Brain aging in acquired immunodeficiency syndrome: increased ubiquitin–protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirology 10:98–108. doi:10.1080/13550280490279816
Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19:407–411. doi:10.1097/01.aids.0000161770.06158.5c
Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M (2003) HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication. J Biosci 28:323–335. doi:10.1007/BF02970151
Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC (2000) Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med 6:143–150. doi:10.1038/77399
Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE (1996) Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem 271:4077–4081. doi:10.1074/jbc.271.8.4077
Lehmann MH, Masanetz S, Kramer S, Erfle V (2006) HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci 119:4520–4530. doi:10.1242/jcs.03231
Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ (2000) Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med 6:1380–1387. doi:10.1038/82199
Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155:853–862
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46:860–866 doi:10.1002/1531-8249(199912)46:6<860::AID-ANA8>3.0.CO;2-M
Pulliam L, Herndier BG, Tang NM, McGrath MS (1991) Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest 87:503–512. doi:10.1172/JCI115024
Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ (1998) Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem 273:32730–32738. doi:10.1074/jbc.273.49.32730
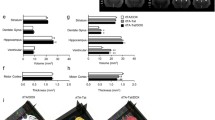
Rempel HC, Pulliam L (2005) HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 19:127–135. doi:10.1097/00002030-200501280-00004
Rogers J, Lue LF (2001) Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int 39:333–340. doi:10.1016/S0197-0186(01)00040-7
Rogers J, Strohmeyer R, Kovelowski CJ, Li R (2002) Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia 40:260–269. doi:10.1002/glia.10153
Selkoe DJ (1998) The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol 8:447–453. doi:10.1016/S0962-8924(98)01363-4
Selkoe DJ (2004) Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med 140:627–638
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14:837–842. doi:10.1038/nm1782
Acknowledgement
The author thanks the Advanced Bioscience Resource for the brain tissue and the National Institutes of Health for their support (LP-RO1MH068213). The author thanks Dr. Hans Rempel, Dr. Bing Sun, Cyrus Calosing and Laure Blouin for technical expertise and editorial comments.