Abstract
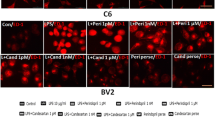
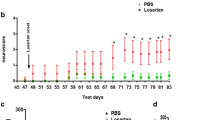
Angiotensin converting enzyme (ACE) converts Angiotensin I to a potent vasoconstrictor angiotensin II (ANG II). ACE inhibitors (ACEIs) are widely used for the management of hypertension. All components of the renin-angiotensin system (RAS) have also been identified in the brain. In addition to cytokines, neuromodulators such as ANG II can induce neuroinflammation. Moreover, in Alzheimer’s disease (AD) models, where neuroinflammation occurs and is thought to contribute to the propagation of the disease, increased levels of ANG II and ACE have been detected. However, the specific effect of ACEIs on neuroinflammation and AD remains obscure. The present study suggests that captopril and perindopril, centrally active ACEIs, may serve as modulators for microglial activation associated with AD. Our in vitro study investigated the effect of both ACEIs on nitric oxide (NO), tumor necrosis factor- α (TNF-α) release and inducible NO synthase (iNOS) expression in lipopolysaccharide (LPS)-induced BV2 microglia. Exposure of BV2 microglia to ACEIs significantly attenuated the LPS-induced NO and TNF-α release. In vivo, short term intranasal administration of perindopril or captopril to 5 Familial AD (5XFAD) mice significantly reduced amyloid burden and CD11b expression (a microglial marker) or only CD11b expression respectively, in the cortex of 5XFAD. Long-term intranasal administration of captopril to mice reduced amyloid burden with no effect on CD11b expression. We provide evidence that intranasal delivery of ACEI may serve as an efficient alternative for their systemic administration, as it results in the attenuation of microglial accumulation and even the reduction of Amyloid β (Aβ) plaques.








Similar content being viewed by others
References
AbdAlla S, El Hakim A, Abdelbaset A, Elfaramawy Y, Quitterer U (2015) Inhibition of ace retards tau hyperphosphorylation and signs of neuronal degeneration in aged rats subjected to chronic mild stress. BioMed Res Int 2015:917156. doi:10.1155/2015/917156
Acharya KR, Sturrock ED, Riordan JF, Ehlers MR (2003) Ace revisited: a new target for structure-based drug design. Nat Rev Drug Dis 2:891–902. doi:10.1038/nrd1227
Akiyama H et al. (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421
Andersson P, Cederholm T, Johansson AS, Palmblad J (2002) Captopril-impaired production of tumor necrosis factor-alpha-induced interleukin-1beta in human monocytes is associated with altered intracellular distribution of nuclear factor-kappaB. J Lab Clin Med 140:103–109
Bhat SA, Goel R, Shukla R, Hanif K (2015) Angiotensin receptor blockade modulates nfkappab and stat3 signaling and inhibits glial activation and neuroinflammation better than angiotensin-converting enzyme inhibition. Mol Neurobiol. doi:10.1007/s12035–015–9584-5
Brown GC (2007) Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans 35:1119–1121. doi:10.1042/bst0351119
Campbell DJ (1987) Circulating and tissue angiotensin systems. J Clin Invest 79:1–6. doi:10.1172/jci112768
Dhuria SV, Hanson LR, Frey WH 2nd (2010) Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99:1654–1673. doi:10.1002/jps.21924
Dong YF et al. (2011) Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 25:2911–2920. doi:10.1096/fj.11-182873
Eckman EA et al. (2006) Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem 281:30471–30478. doi:10.1074/jbc.M605827200
Fu H et al. (2012) Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Abeta by microglia. Glia 60:993–1003. doi:10.1002/glia.22331
Galasko DR et al. (2012) Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol 69:836–841. doi:10.1001/archneurol.2012.85
Gibbons HM, Dragunow M (2006) Microglia induce neural cell death via a proximity-dependent mechanism involving nitric oxide. Brain Res 1084:1–15. doi:10.1016/j.brainres.2006.02.032
Good PF, Werner P, Hsu A, Olanow CW, Perl DP (1996) Evidence of neuronal oxidative damage in Alzheimer’s disease. Am J Pathol 149:21–28
Haas J, Storch-Hagenlocher B, Biessmann A, Wildemann B (2002) Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with beta-amyloid 1-42 in vitro. Neurosci Lett 322:121–125
Hartlage-Rubsamen M, Lemke R, Schliebs R (1999) Interleukin-1beta, inducible nitric oxide synthase, and nuclear factor-kappaB are induced in morphologically distinct microglia after rat hippocampal lipopolysaccharide/interferon-gamma injection. J Neurosci Res 57:388–398
Hemming ML, Selkoe DJ (2005) Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 280:37644–37650. doi:10.1074/jbc.M508460200
Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA (1998) Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. The journal of neuroscience: the official journal of the society for. Neuroscience 18:8126–8132
Holscher C (2014) First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer’s disease Alzheimer’s & dementia. J Alzheimer’s Association 10:S33–S37. doi:10.1016/j.jalz.2013.12.006
Hou DR et al. (2008) Altered angiotensin-converting enzyme and its effects on the brain in a rat model of Alzheimer disease. Chin Med J 121:2320–2323
Hu J, Igarashi A, Kamata M, Nakagawa H (2001) Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta ); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem 276:47863–47868. doi:10.1074/jbc.M104068200
Hung AS, Liang Y, Chow TC, Tang HC, Wu SL, Wai MS, Yew DT (2016) Mutated tau, amyloid and neuroinflammation in Alzheimer disease-A brief review. Prog Histochem Cytochem. doi:10.1016/j.proghi.2016.01.001
Inagami T, Kambayashi Y, Ichiki T, Tsuzuki S, Eguchi S, Yamakawa T (1999) Angiotensin receptors: molecular biology and signalling. Clin Exp Pharmacol Physiol 26:544–549
Karamyan VT, Speth RC (2007) Enzymatic pathways of the brain renin-angiotensin system: unsolved problems and continuing challenges. Regul Pept 143:15–27. doi:10.1016/j.regpep.2007.03.006
Khachaturian AS et al. (2006) Antihypertensive medication use and incident Alzheimer disease: the cache county study. Arch Neurol 63:686–692. doi:10.1001/archneur.63.5.noc60013
Kumar, A, Chen, SH, Kadiiska, MB, Hong, JS, Zielonka, J, Kalyanaraman, B, Mason, RP (2014) Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells. Free Radical Biol Med 73:51–59 doi:10.1016/j.freeradbiomed.2014.04.014
Lavoie JL, Cassell MD, Gross KW, Sigmund CD (2004) Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension 43:1116–1119. doi:10.1161/01.hyp.0000125143.73301.94
Laws SM et al. (2005) TNF polymorphisms in Alzheimer disease and functional implications on CSF beta-amyloid levels. Hum Mutat 26:29–35. doi:10.1002/humu.20180
Lieberman AP, Pitha PM, Shin HS, Shin ML (1989) Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA 86(16):6348–6352
Lovell MA, Markesbery WR (2007) Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res 35:7497–7504. doi:10.1093/nar/gkm821
Maezawa I, Zimin PI, Wulff H, Jin LW (2011) Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J Biol Chem 286:3693–3706. doi:10.1074/jbc.M110.135244
Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vazquez CM (2010) Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB Journal of inflammation (London, England) 7:21 doi:10.1186/1476-9255-7-21
Miners JS, Barua N, Kehoe PG, Gill S, Love S (2011) Abeta-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol 70:944–959. doi:10.1097/NEN.0b013e3182345e46
Nimmo AJ, Vink R (2009) Recent patents in CNS drug discovery: the management of inflammation in the central nervous system. Recent Pat CNS Drug Discov 4:86–95
Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA (2006) Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol 65:631–641. doi:10.1097/01.jnen.0000228136.58062.bf
O’Caoimh R et al. (2014) Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. Journal of Alzheimer’s disease : JAD 40:595–603. doi:10.3233/jad-131694
Oakley H et al. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:10129–10140. doi:10.1523/jneurosci.1202-06.2006
Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H (2005) The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide. Eur J Neurosci 21:733–740. doi:10.1111/j.1460–9568.2005.03912.x
Ohrui T et al. (2004) Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 63:1324–1325
Peach MJ (1977) Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57:313–370
Qadri F et al. (2015) Acute hypothalamo-pituitary-adrenal axis response to LPS-induced endotoxemia: expression pattern of kinin type B1 and B2 receptors. Biol Chem. doi:10.1515/hsz-2015-0206
Rangasamy SB et al. (2015) Intranasal delivery of nemo-binding domain peptide prevents memory loss in a mouse model of Alzheimer’s disease. J Alzheimer’s Dis: JAD 47:385–402. doi:10.3233/jad-150040
Reynolds MR, Reyes JF, Fu Y, Bigio EH, Guillozet-Bongaarts AL, Berry RW, Binder LI (2006) Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer’s disease and other tauopathies. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:10636–10645. doi:10.1523/jneurosci.2143-06.2006
Righi M et al. (1989) Monokine production by microglial cell clones. Eur J Immunol 19(8):1443–1448. doi:10.1002/eji.1830190815
Sano M et al. (1997) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s disease cooperative study. N Engl J Med 336:1216–1222. doi:10.1056/nejm199704243361704
Savaskan E, Hock C, Olivieri G, Bruttel S, Rosenberg C, Hulette C, Muller-Spahn F (2001) Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer’s dementia. Neurobiol Aging 22:541–546
Shadfar S, Hwang CJ, Lim MS, Choi DY, Hong JT (2015) Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Arch Pharmacal Res 38:2106–2119. doi:10.1007/s12272–015-0648-x
Simic G, Lucassen PJ, Krsnik Z, Kruslin B, Kostovic I, Winblad B, Bogdanovi (2000) nNOS expression in reactive astrocytes correlates with increased cell death related DNA damage in the hippocampus and entorhinal cortex in Alzheimer’s disease. Exp Neurol 165:12–26. doi:10.1006/exnr.2000.7448
Sink KM et al. (2009) Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch Intern Med 169:1195–1202. doi:10.1001/archinternmed.2009.175
Skrbic R, Igic R (2009) Seven decades of angiotensin (1939-2009. Peptides 30:1945–1950. doi:10.1016/j.peptides.2009.07.003
Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G (1997) Widespread peroxynitrite-mediated damage in Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 17:2653–2657
Solfrizzi V et al. (2013) Angiotensin-converting enzyme inhibitors and incidence of mild cognitive impairment. The Italian longitudinal study on aging. Age (Dordr) 35:441–453. doi:10.1007/s11357-011-9360-z
Song K, Allen AM, Paxinos G, Mendelsohn FA (1992) Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol 316:467–484. doi:10.1002/cne.903160407
Tarkowski E, Andreasen N, Tarkowski A, Blennow K (2003a) Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:1200–1205
Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K (2003b) Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull 61:255–260
Thorns V, Hansen L, Masliah E (1998) nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer’s disease. Exp Neurol 150:14–20. doi:10.1006/exnr.1997.6751
Tohgi H, Abe T, Yamazaki K, Murata T, Ishizaki E, Isobe C (1999) Alterations of 3-nitrotyrosine concentration in the cerebrospinal fluid during aging and in patients with Alzheimer’s disease. Neurosci Lett 269:52–54
Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J (2003) Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 163:1069–1075. doi:10.1001/archinte.163.9.1069
Viel TA, Buck HS (2011) Kallikrein-kinin system mediated inflammation in Alzheimer’s disease in vivo. Current Alzheimer research 8:59–66
Wright JW, Kawas LH, Harding JW (2013) A Role for the Brain RAS in Alzheimer’s and Parkinson’s. Dis Frontiers Endocrinol 4:158. doi:10.3389/fendo.2013.00158
Yamada K et al. (2010) Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer’s disease. Brain Res 1352:176–186. doi:10.1016/j.brainres.2010.07.006
Acknowledgments
We thank Prof. Rosario Donato (Department of Experimental Medicine and Biochemical Sciences, University of Perugia, Italy) for providing us the BV2 murine microglial cell line.
Five familial alzheimer’s disease mice were kindly provided by Prof. Robert Vassar (Department of Cell and Molecular Biology, Northwestern University, Chicago, Illinois).
Rabbit anti human Aβ antibody was received as a gift from Prof. Alon Monsonego, (The Shraga Segal Department of Microbiology and Immunology, Faculty of Health Sciences and the National Institute of Biotechnology in the Negev, Ben-Gurion University of the Negev, Beer-Sheva, Israel).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Israel Science Foundation (ISF) grant to Sigal Fleisher-Berkovich no: 101/11–16.
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Nofar Torika and Keren Asraf equally contributed to this paper
This research was supported by the Israel Science Foundation (grant No 101/11–16)
Rights and permissions
About this article
Cite this article
Torika, N., Asraf, K., Roasso, E. et al. Angiotensin Converting Enzyme Inhibitors Ameliorate Brain Inflammation Associated with Microglial Activation: Possible Implications for Alzheimer’s Disease. J Neuroimmune Pharmacol 11, 774–785 (2016). https://doi.org/10.1007/s11481-016-9703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9703-8