Abstract
Radiation is commonly used in cancer treatment. Over 50% of all cancer patients will undergo radiotherapy (RT) as part of cancer care. Scientific advances in RT have primarily focused on the physical characteristics of treatment including beam quality and delivery. Only recently have inroads been made into utilizing tumor biology and radiobiology to design more appropriate RT protocols. Tumors are composites of proliferating and growth-arrested cells, and overall response depends on their respective proportions at irradiation. Prokopiou et al. (Radiat Oncol 10:159, 2015) developed the concept of the proliferation saturation index (PSI) to augment the clinical decision process associated with RT. This framework is based on the application of the logistic equation to pre-treatment imaging data in order to estimate a patient-specific tumor carrying capacity, which is then used to recommend a specific RT protocol. It is unclear, however, how dependent clinical recommendations are on the underlying tumor growth law. We discuss a PSI framework with a generalized logistic equation that can capture kinetics of different well-known growth laws including logistic and Gompertzian growth. Estimation of model parameters on the basis of clinical data revealed that the generalized logistic model can describe data equally well for a wide range of the generalized logistic exponent value. Clinical recommendations based on the calculated PSI, however, are strongly dependent on the specific growth law assumed. Our analysis suggests that the PSI framework may best be utilized in clinical practice when the underlying tumor growth law is known, or when sufficiently many tumor growth models suggest similar fractionation protocols.








Similar content being viewed by others
References
Ahmed KA, Correa CR, Dilling T, Caudell JJ (2014) Altered fractionation schedules in radiation treatment: a review. Semin Oncol 41(6):730–750
Alfonso JCL, Buttazzo G, Garcia-Archilla B, Herrero M, Nunez L (2014) Selecting radiotherapy dose distributions by means of constrained optimization problems. Bull Math Biol 76(5):1017–1044
Allison RR, Patel RM, McLawhorn RA (2014) Radiation oncology: physics advances that minimize morbidity. Future Oncol 10(15):2329–2344
Anderson AR, Quaranta V (2008) Integrative mathematical oncology. Nat Rev Cancer 8(3):227–234
Benzekry S, Lamont C, Beheshti A, Tracz A, Ebos JM, Hlatky L, Hahnfeldt P (2014) Classical mathematical models for description and prediction of experimental tumor growth. PLoS Comput Biol 10(8):e1003800
Bourhis J, Overgaard J, Audry H, Ang K, Saunders M, Bernier J, Horiot J, Le Maitre A, Pajak T, Poulsen M, O’Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay J, Pinto L, Fallai C, Fu K, Sylvester R (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368(9538):843–854
Byrne HM, Chaplain MA (1995) Growth of nonnecrotic tumors in the presence and absence of inhibitors. Math Biosci 130(2):151–181
Choe SC, Zhao G, Zhao Z, Rosenblatt JD, Cho HM, Shin SU, Johnson NF (2011) Model for in vivo progression of tumors based on co-evolving cell population and vasculature. Sci Rep 1:31
Deisboeck TS, Wang Z (2007) Cancer dissemination: a consequence of limited carrying capacity? Med Hypotheses 69(1):173–177
Enderling H, Chaplain MA, Hahnfeldt P (2010) Quantitative modeling of tumor dynamics and radiotherapy. Acta Biotheor 58(4):341–353
Enderling H, Park D, Hlatky L, Hahnfeldt P (2009) The importance of spatial distribution of stemness and proliferation state in determining tumor radioresponse. Math Model Nat Phenom 4(03):117–133
Folkman J, Hochberg M (1973) Self-regulation of growth in three dimensions. J Exp Med 138(4):745–753
Fowler J (2014) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62:679–694
Fowler JF (2010) 21 years of biologically effective dose. Br J Radiol 83(991):554–568
Gao X, McDonald JT, Hlatky L, Enderling H (2013) Acute and fractionated irradiation differentially modulate glioma stem cell division kinetics. Cancer Res 73(5):1481–1490
Gerlee P (2013) The model muddle: in search of tumor growth laws. Cancer Res 73(8):2407–2411
Gerlee P, Anderson AR (2015) The evolution of carrying capacity in constrained and expanding tumour cell populations. Phys Biol 12(5):056001
Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L (1999) Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res 59(19):4770–4775
Hillen T, Enderling H, Hahnfeldt P (2013) The tumor growth paradox and immune system-mediated selection for cancer stem cells. Bull Math Biol 75(1):161–184
Hubenak JR, Zhang Q, Branch CD, Kronowitz SJ (2015) Mechanisms of injury to normal tissue after radiotherapy: a review. Plastic Reconstr Surg 133(1):49e–56e
Kempf H, Bleicher M, Meyer-Hermann M (2015) Spatio-temporal dynamics of hypoxia during radiotherapy. PLoS One 10(8):e0133357
Laperriere NJ, Bernstein M (1994) Radiotherapy for brain tumors. CA Cancer J Clin 44(2):196108
Murphy H, Jaafari H, Dobrovolny HM (2016) Differences in predictions of ODE models of tumor growth: a cautionary example. BMC Cancer 16(1):163
Poleszczuk J, Luddy KA, Prokopiou S, Robertson-Tessi M, Moros EG, Fishman M, Djeu J, Finkelstein SE, Enderling H (2016) Abscopal benefits of localized radiotherapy depend on activated t cell trafficking and distribution between metastatic lesions. Cancer Res 76(5):1009–1018
Powathil GG, Adamson DJ, Chaplain MA (2013) Towards predicting the response of a solid tumour to chemotherapy and radiotherapy treatments: clinical insights from a computational model. PLoS Comput Biol 9(7):e1003120
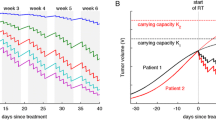
Prokopiou S, Moros EG, Poleszczuk J, Caudell J, Torres-Roca JF, Latifi K, Lee JK, Myerson R, Harrison LB, Enderling H (2015) A proliferation saturation index to predict radiation response and personalize radiotherapy fractionation. Radiat Oncol 10:159
Qiao X, Tullgren O, Sirzen F, Lewenshon R (2003) The role of radiotherapy in treatment of stage i non-small cell lung cancer. Lung Cancer 41(1):1–11
Ribba B, Colin T, Schnell S (2006) A multiscale mathematical model of cancer, and its use in analyzing irradiation therapies. Theor Biol Med Model 3:7
Richards F (1959) A flexible growth function for empirical use. J Exp Bot 10(2):290–300
Rockne RC, Rockhill JK, Mrugala M, Spence A, Kalet I, Hendrickson K, Lai A, Cloughesy T, Alvord E Jr, Swanson KR (2010) Predicting the efficacy of radiotherapy in individual glioblastoma patients in vivo: a mathematical modeling approach. Phys Med Biol 55(12):3271–3285
Roose T, Chapman SJ, Maini PK (2007) Mathematical models of avascular tumor growth. SIAM Rev 49(2):179–208
Saunders M, Rojas A, Parmar M, Dische S, Collaborators C (2010) Mature results of a randomized trial of accelerated hyperfractionated versus conventional radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Phys 77:3–8
Sharma S, Bekelman J, Lin A, Lukens NJ, Roman BR, Mitra N, Swisher-McClure S (2016) Clinical impact of prolonged diagnosis to treatment interval (dti) among patients with oropharyngeal squamous cell carcinoma. Oral Oncol 56:17–24
Stevens C, Bondy SJ, Loblaw AD (2010) Wait times in prostate cancer diagnosis and radiation treatment. Can Urol Assoc J 4(4):243–248
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncol 9(S5):4–9
Wang L, Correa CR, Hayman JA, Zhao L, Cease K, Brenner D, Arenberg D, Curtis J, Kalemkerian GP, Kong FM (2009) Time to treatment in patients with stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 74(3):790–795
Wang P, Feng Y (2013) A mathematical model of tumor volume changes during radiotherapy. Sci World J 4:1322–1330
Whelan TJ (2005) Use of conventional radiation therapy as part of breast-conserving treatment. J Clin Oncol 23(8):1718–1725
Yankeelov TE, Quaranta V, Evans KJ, Rericha EC (2015) Toward a science of tumor forecasting for clinical oncology. Cancer Res 75(6):918–923
Yoshimura M, Itasaka S, Harada H, Hiraoka M (2013) Microenvironment and radiation therapy. BioMed Res Int 2013:685308
Acknowledgements
This work is supported in part by the Personalized Medicine Award 09-33000-15-03 from the DeBartolo Family Personalized Medicine Institute Pilot Research Awards in Personalized Medicine (PRAPM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poleszczuk, J., Walker, R., Moros, E.G. et al. Predicting Patient-Specific Radiotherapy Protocols Based on Mathematical Model Choice for Proliferation Saturation Index. Bull Math Biol 80, 1195–1206 (2018). https://doi.org/10.1007/s11538-017-0279-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0279-0