Summary
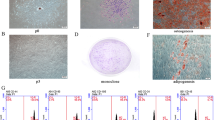
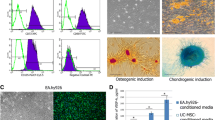
By co-culturing humm mesenchymal stem cells (hMSCs) and human umbilical rein endothelial cells (HUVECs) under hypoxia and creating a microenvironment similar to that of transplanted hMSCs for the treatment of avascular ni ANFH, the effect of hMSCs on survival, apoptosis, migration and angiogenesis of human umbilical vein endothelial cells (HUVECs) under the hypoxic condition were investigated in vitro. hMSCs and HUVECs were cultured and identified in vitro. Three kinds of conditioned media, CdM-CdMNOR, CdM-CdMHYP and HUVEC-CdMHYP were prepared. HUVECs were cultured with these conditioned media under hypoxia. The survival rate, apoptosis rate, migration and angiogenesis of HUVECs were respectively detected by CCK-8, flow cytometry, Transwell and tube formation assay. The content of SDF-1α, VEGF and IL-6 in CdM was determined by ELISA. Our results showed that hMSCs and HUVECs were cultured and identified successfully. Compared with MSC-CdMNOR and HUVEC-CdMHYP groups, the survival rate, migration and angiogenesis of HUVECs in MSC-CdMHYP group were significantly increased while the apoptosis rate was declined (P<0.05). Moreover, the expression of SDF-1α, VEGF and IL-6 in MSC-CdMHYP group was up-regulated. Under hypoxia, the apoptosis of HUVECs was inhibited while survival, migration and angiogenesis were improved by co-culture of hMSCs and HUVECs. The underlying mechanism may be that hMSCs could secrete a number of cytokines and improve niche, which might be helpful in the treatment of femoral head necrosis.
Similar content being viewed by others
References
NReis ND, Schwartz O, Militianu D, et al. Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br, 2003,85(3):371–375
Alves EM, Angrisani AT, Santiago MB. The use of extracorporeal shock waves in the treatment of osteonecrosis of the femoral head: a systematic review. Clin Rheumatol, 2009,28(11):1247–1251
Lai KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am, 2005,87(10):2155–2159
Castro FP Jr, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop (Belle Mead NJ). 2000,29(3):187–194
Nakamura Y, Kumazawa Y, Mitsui H, et al. Combined rotational osteotomy and vascularized iliac bone graft for advanced osteonecrosis of the femoral head. J Reconstr Microsurg, 2005,21(2):101–105
Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res, 2004, (429):139–145
Granero-Molto F, Weis JA, Longobardi L, et al. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther, 2008,8(3):255–268
Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009,27(8):1887–1898
Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med, 1999,5(3):309–313
Cui Q, Xiao Z, Li X, et al. Use of genetically engineered bone-marrow stem cells to treat femoral defects: an experimental study. J Bone Joint Surg Am, 2006,88(Suppl 3):167–172
Yan Z, Hang D, Guo C, et al. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res, 2009,27(4):442–6.
Hung SC, Pochampally RR, Chen SC, et al. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells, 2007,25(9):2363–2370
Ikenaga S, Hamano K, Nishida M, et al. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J Surg Res, 2001,96(2):277–83
Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet, 2002,360(9331):427–435
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science, 1999,284(5411):143–147
Owen M. Marrow stromal stem cells. J Cell Sci Suppl, 1988,10:63–76
Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells, 2007,25(11):2896–2902
Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther, 2005,5(4):437–442
Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am, 2005,87(Suppl 1) (Pt 1):106–112
Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res, 2002, (405):14–23
Cui Q, Xiao Z, Li X, et al. Use of genetically engineered bone-marrow stem cells to treat femoral defects: an experimental study. J Bone Joint Surg Am, 2006,88(Suppl 3):167–172
Ji WF, Tong PJ, Zheng WB, et al. Experimental study on treatment of femoral head necrosis with arterial perfusion of marrow stem cells]. Zhongguo Zhong Xi Yi Jie He Za Zhi (Chinese), 2004,24(11):999–1002
Li ZH, Liao W, Cui XL, et al. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci, 2011,8(1):74–83
Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA, 2002,99(13):8932–8937
Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg, 2005,80(1):229–236; discussion 236–237
Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med, 2005, 11(4):367–368
Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells, 2008,26(1):151–162
Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med, 2004,10(8):858–864
Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science, 1999,283(5403):845–848
Liu H, Pan Z, Li A, et al. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol, 2008,5(5):373–378
Potier E, Ferreira E, Andriamanalijaona R, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone, 2007,40(4):1078–1087
Muller I, Vaegler M, Holzwarth C, et al. Secretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosis. Leukemia. 2008,22(11):2054–2061
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from the National Natural Sciences Foundation of China (No. 30750010).
Rights and permissions
About this article
Cite this article
Zhang, B., Yang, S., Zhang, Y. et al. Co-culture of mesenchymal stem cells with umbilical vein endothelial cells under hypoxic condition. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 173–180 (2012). https://doi.org/10.1007/s11596-012-0031-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-0031-9