Summary
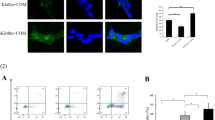
The vitamin K epoxide reductase complex subunit 1 (VKORC1), the rate-limiting enzyme for vitamin K recycling, is significantly down-regulated in the kidneys of urolithiasis patients. This study searched for direct evidence to define the inhibitory activity of VKORC1 against calcium oxalate (CaOx) crystal formation. In the experiment of VKORC1 overexpression, HK-2 cells were transfected with the pFLAG-CMV-7.1-VKORC1 plasmid as a pFLAG-CMV-7.1-VKORC1 transfection group or the pFLAG-CMV-7.1 plasmid as a pFLAG-CMV-7.1 control group. In the experiment of VKORC1 knockdown, HK-2 cells were transfected with the PGPU6/GFP/Neo-VKORC1shRNA-2 as a PGPU6/GFP/Neo-VKORC1shRNA-2 transfection group or the PGPU6/GFP/Neo-shRNA-NC plasmid as a PGPU6/GFP/Neo-shRNA-NC control group. The expression of VKORC1 in HK-2 cells was detected by real-time quantitative PCR and Western blotting. The CaOx crystal formation was observed under the laser-scanning confocal microscope. It was found that the expression levels of VKORC1 mRNA and protein were significantly higher in the pFLAG-CMV-7.1-VKORC1 transfection group than in the pFLAG-CMV-7.1 control group (P<0.01). The number of CaOx crystals in HK-2 cells incubated in fluorescently labeled CaOx monohydrate (COM) crystal medium for 48 h was 14±4 per field (100×) in the pFLAG-CMV-7.1-VKORC1 transfection group and 26±5 per field (100×) in the pFLAG-CMV-7.1 control group respectively under the laser-scanning confocal microscope. The amount of CaOx crystal aggregation and formation in the pFLAG-CMV-7.1-VKORC1 transfection group was significantly reduced as compared with the pFLAG-CMV-7.1 control group (P<0.05). The expression levels of VKORC1 mRNA and protein were significantly lower in the PGPU6/GFP/Neo-VKORC1shRNA-2 transfection group than in the PGPU6/GFP/Neo-shRNA-NC control group (P<0.05). The number of CaOx crystals in HK-2 cells incubated in fluorescently labeled COM crystal medium was 65±11 per field (100×) in the PGPU6/GFP/Neo-VKORC1shRNA-2 transfection group and 24±6 per field (100×) in the PGPU6/GFP/Neo-shRNA-NC control group respectively under the laser-scanning confocal microscope. The amount of CaOx crystal aggregation and formation in the PGPU6/GFP/Neo-VKORC1shRNA-2 transfection group was significantly increased as compared with the PGPU6/GFP/Neo-shRNA-NC control group (P<0.05). These findings suggested that the VKORC1 protein could inhibit CaOx salt crystallization, adhesion and aggregation. This research would help us to understand the mechanisms involving the interaction between crystallization and epithelial cells and the formation of CaOx.
Similar content being viewed by others
References
Moe OW. Kidney stones: pathophysiology and medical management. Lancet, 2006, 367(9507):333–344
Worcester EM, Coe FL. Clinical practice: calcium kidney stones. N Engl J Med, 2010, 363(10):954–963
Verkoelen CF, Verhulst A. Proposed mechanisms in renal tubular crystal retention. Kidney Int, 2007, 72(1):13–18
Hess B, Nakagawa Y, Parks JH, et al. Molecular abnormality of Tamm-Horsfall glycoprotein in calcium oxalate nephrolithiasis. Am J Physiol, 1991, 260(4 Pt 2):F569–578
Asplin JR, Arsenault D, Parks JH, et al. Contribution of human uropontin to inhibition of calcium oxalate crystallization. Kidney Int, 1998, 53(1):194–199
Pillay SN, Asplin JR, Coe FL. Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am J Physiol, 1998, 275(2 Pt 2):F255–261
Atmani F, Khan SR. Characterization of uronic-acid-rich inhibitor of calcium oxalate crystallization isolated from rat urine. Urol Res, 1995, 23(2):95–101
Stapleton AM, Ryall RL. Blood coagulation proteins and urolithiasis are linked: crystal matrix protein is the F1 activation peptide of human prothrombin. Br J Urol, 1995, 75(6):712–719
Hu B, Wang T, Liu Z, et al. Decreased expression of vitamin K epoxide reductase complex subunit 1 in kidney of patients with calcium oxalate urolithiasis. J Huazhong Univ Sci Technolog Med Sci, 2011, 31(6):807–814
Liu J, Chen J, Wang T, et al. Effects of urinary prothrombin fragment 1 in the formation of calcium oxalate calculus. J Urol, 2005, 173(1):113–116
Gong X, Gutala R, Jaiswal AK. Quinone oxidoreductases and vitamin K metabolism. Vitam Horm, 2008, 78(1): 85–101
Chaiyarit S, Mungdee S, Thongboonkerd B. Non-radioactive labelling of calcium oxalate crystals for investigations of crystal-cell interactions and internalization. Anal Methods, 2010, 2(10):1536–1541
Verkoelen CF. Crystal retention in renal stone disease: a crucial role for the glycosaminoglycan hyaluronan. J Am Soc Nephrol, 2006, 17(6):1673–1687
Khan SR, Byer KJ, Thamilselvan S, et al. Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J Am Soc Nephrol, 1999, 10(14): S457–463
Lieska JC, Toback FG. Renal cell-urinary crystal interactions. Curr Opin Nephrol Hypertens, 2000, 9(4):349–355
Wiessner JH, Hasegawa AT, Hung LY, et al. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int, 2001, 59(2):637–644
Hovda KE, Guo C, Austin R, et al. Renal toxicity of ethylene glycol results from internalization of calcium oxalate crystals by proximal tubule cells. Toxicol Lett, 2010, 192(3):365–372
Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int, 1994, 46(3):847–854
Parmar MS. Kidney stones. BMJ, 2004, 328(7453): 1420–1424
Worcester EM. Inhibitors of stone formation. Semin Nephrol, 1996, 16(5): 474–486
Stafford DW. The vitamin K cycle. J Thromb Haemost, 2005, 3(8):1873–1878
Ryall RL, Grover PK, Stapleton AM, et al. The urinary FI activation peptide of human prothrombin is a potent inhibitor of calcium oxalate crystallization in undiluted human urine in vitro. Clin Sci (Lond), 1995, 89(5): 533–541
Grover PK, Ryall RL. Effect of prothrombin and its activation fragments on calcium oxalate crystal growth and aggregation in undiluted human urine in vitro: relationship between protein structure and inhibitory activity. Clin Sci (Lond), 2002, 102(4):425–434
Grover PK, Ryall RL. Inhibition of calcium oxalate crystal growth and aggregation by prothrombin and its fragments in vitro: relationship between protein structure and inhibitory activity. Eur J Biochem, 1999, 263(1):50–56
Grover PK, Moritz RL, Simpson RJ, et al. Inhibition of growth and aggregation of calcium oxalate crystals in vitro—a comparison of four human proteins. Eur J Biochem, 1998, 253(3):637–644
Author information
Authors and Affiliations
Corresponding author
Additional information
Both authors contributed equally to this work.
This project was supported by grants from the Scientific Research Starting Foundation for Young Scientist from Shanghai Medical College of Fudan University (No. 11L-33) and the Shanghai Municipal Key Specialist Construction Projects (No. ZK2012A22).
Rights and permissions
About this article
Cite this article
Hu, B., Wu, Hr., Ma, Zy. et al. Involvement of VKORC1 in the inhibition of calcium oxalate crystal formation in HK-2 cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 376–381 (2014). https://doi.org/10.1007/s11596-014-1286-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1286-0