Abstract
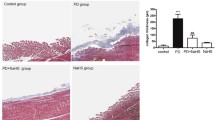
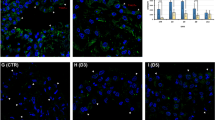
The objective of this study was to investigate the role of the RhoA/Rock signaling pathway in the epithelial–mesenchymal transition (EMT) of rat peritoneal mesothelial cells (RPMCs). Primary SD rat peritoneal mesothelial cells were cultured in vitro. RPMCs were randomly assigned to four groups: group A (control), group B (TGF-β1, 10 μg/L), group C (10 μg/L TGF-β1 + 10 μmol/L Y-27632, an inhibitor of Rock that was pre-applied for 2 h before TGF-β1 stimulation), and group D (Y-27632 alone, 10 μmol/L). Our results were as follows: (1) TGF-β1 stimulation elicited a robust increase in RhoA activity in a time-dependent manner; the increase was 2.57 ± 0.52 times larger than the activity observed for the control group (P < 0.05) after 10 min of stimulation. RhoA activity peaked at 1 h and was 4.35 ± 0.41 times the value observed for the control group (P < 0.05). (2) TGF-β1 up-regulated mRNA and/or protein expression of α-SMA, vimentin, and collagen and down-regulated mRNA and protein expression of E-cadherin in RPMCs. (3) The Rock inhibitor Y-27632 effectively reduced TGF-β1-induced expression of α-SMA, collagen, and vimentin; the mRNA levels of α-SMA and collagen decreased by 53.8% and 55.7%, respectively, and the protein levels of α-SMA, vimentin, and collagen decreased by 42.6%, 60.1%, and 58.1%, respectively, as compared to TGF-β1-stimulated groups (P < 0.05). However, the Rock inhibitor Y-27632 had no effect on the level of E-cadherin. In conclusion, the RhoA/Rock signaling pathway may mediate EMT induced by TGF-β1 in rat peritoneal mesothelial cells. The RhoA/Rock pathway may be a potential therapeutic target for the treatment of peritoneal fibrosis.








Similar content being viewed by others
References
Betjes M. G.; Bos H. J.; Krediet R. T.; Arisz L. The mesothelial cells in CAPD effluent and their relation to peritonitis incidence. Perit. Dial. Int. 11: 22–26; 1991.
Bishop A. L.; Hall A. Rho GTPases and their effector proteins. Biochem. J. 348(Pt 2): 241–255; 2000.
Burridge K.; Wennerberg K. Rho and Rac take center stage. Cell 116: 167–179; 2004.
Dou X. R.; Yu W. Q.; Hao W. K.; Nie J.; Li X. Y.; Chen W. F.; Wang X.; Jia Z. J. Smad7 overexpression inhibits epithelial–mesenchymal transition in peritoneal fibrosis rat model. Chin. J. Nephrol. 22: 612–616; 2006.
Essig M.; Vrtovsnik F.; Friedlander G. Inhibitors of HMG CoA reductase: new modes of action, new indications? Therapie 55: 43–49; 2000.
Gotloib L.; Wajsbrot V.; Cuperman Y.; Shostak A. Acute oxidative stress induces peritoneal hyperpermeability, mesothelial loss, and fibrosis. J. Lab. Clin. Med. 143: 31–40; 2004.
Jiang Z.; Seo J. Y.; Ha H.; Lee E. A.; Kim Y. S.; Han D. C.; Uh S. T.; Park C. S.; Lee H. B. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem. Biophys. Res. Commun. 309: 961–966; 2003.
Leavesley D. I.; Stanley J. M.; Faull R. J. Epidermal growth factor modifies the expression and function of extracellular matrix adhesion receptors expressed by peritoneal mesothelial cells from patients on CAPD. Nephrol. Dial. Transplant. 14: 1208–1216; 1999.
Linden T.; Musi B.; Jarkelid L.; Forsback G.; Kjellstrand P.; Deppisch R.; Wieslander A. Glucose degradation products in peritoneal dialysis fluids may have both local and systemic effects: a study of residual fluid and mesothelial cells. Perit. Dial. Int. 21: 607–610; 2001.
Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15: 1–12; 2004.
Murata T.; Arii S.; Mori A.; Imamura M. Therapeutic significance of Y-27632, a Rho-kinase inhibitor, on the established liver fibrosis. J. Surg. Res. 114: 64–71; 2003.
Murata T.; Arii S.; Nakamura T.; Mori A.; Kaido T.; Furuyama H.; Furumoto K.; Nakao T.; Isobe N.; Imamura M. Inhibitory effect of Y-27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. J. Hepatol. 35: 474–481; 2001.
Nagatoya K.; Moriyama T.; Kawada N.; Takeji M.; Oseto S.; Murozono T.; Ando A.; Imai E.; Hori M. Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 61: 1684–1695; 2002.
Patel S.; Takagi K. I.; Suzuki J.; Imaizumi A.; Kimura T.; Mason R. M.; Kamimura T.; Zhang Z. RhoGTPase activation is a key step in renal epithelial mesenchymal transdifferentiation. J. Am. Soc. Nephrol. 16: 1977–1984; 2005.
Rhyu D. Y.; Yang Y.; Ha H.; Lee G. T.; Song J. S.; Uh S. T.; Lee H. B. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial–mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 16: 667–675; 2005.
Sahai E.; Marshall C. J. RHO-GTPases and cancer. Nat. Rev. Cancer 2: 133–142; 2002.
Shimizu Y.; Dobashi K.; Iizuka K.; Horie T.; Suzuki K.; Tukagoshi H.; Nakazawa T.; Nakazato Y.; Mori M. Contribution of small GTPase Rho and its target protein rock in a murine model of lung fibrosis. Am. J. Respir. Crit. Care Med. 163: 210–217; 2001.
Symons M.; Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 10: 415–419; 2000.
Uehata M.; Ishizaki T.; Satoh H.; Ono T.; Kawahara T.; Morishita T.; Tamakawa H.; Yamagami K.; Inui J.; Maekawa M.; Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994; 1997.
Wettschureck N.; Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J. Mol. Med. 80: 629–638; 2002.
Witowski J.; Wisniewska J.; Korybalska K.; Bender T. O.; Breborowicz A.; Gahl G. M.; Frei U.; Passlick-Deetjen J.; Jorres A. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J. Am. Soc. Nephrol. 12: 2434–2441; 2001.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Hao Zhang and Xiaoxian Liu are co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, H., Liu, X., Liu, Y. et al. Epithelial–mesenchymal transition of rat peritoneal mesothelial cells via Rhoa/Rock pathway. In Vitro Cell.Dev.Biol.-Animal 47, 165–172 (2011). https://doi.org/10.1007/s11626-010-9369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-010-9369-0