Abstract
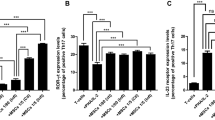
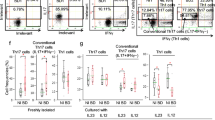
Regarding discrepancies that exist among different studies which have tried to clarify critical factors in human Th17 cell differentiation, the aim of this study was to identify the best condition for human Th17 differentiation and to clarify the possible role of TGF-β in differentiation of these cells. Naïve CD4+ T cells were isolated from cord blood samples and cultured either in X-VIVO 15 serum-free medium or RPMI 1640 containing 10% FBS. Purified cells were treated with different combinations of polarizing cytokines (TGF-β, IL-1β, IL-6, IL-23 and IL-21) followed by analysis of the expression of characteristic genes and their relevant cytokines by real-time quantitative RT-PCR and ELISA method, respectively. Our data indicate that a combination of TGF-β plus IL-6 and IL-23 cytokines in X-VIVO 15 serum-free medium could be applied as the best condition for developing human Th17 cells in compare with other studied cytokine treatments. It is shown that TGF-β could be considered as a positive regulator for human Th17 cell differentiation only if applied in average concentrations. Interestingly, polarizing treatments in absence of TGF-β, induced double-secreting Th17 cells which co-express IL-17 and IFN-γ whereas polarization in presence of TGF-β-induced single-secreting (only IL-17 expressing) Th17 cells.







Similar content being viewed by others
References
Acosta-Rodriguez E. V.; Napolitani G.; Lanzavecchia A.; Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8: 942–949; 2007a.
Acosta-Rodriguez E. V.; Rivino L.; Geginat J.; Jarossay D.; Gattorno M. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8: 639–46; 2007b.
Aggarwal S.; Ghilardi N.; Xie M. H.; de Sauvage F. J.; Gurney A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278: 1910–1914; 2003.
Annunziato F.; Cosmi L.; Liotta F.; Maggi E.; Romagnani S. Human Th17 cells: are they different from murine Th17 cells? Eur. J. Immunol. 39: 637–640; 2009.
Annunziato F.; Cosmi L.; Santarlasci V.; Maggi L.; Liotta F.; Mazzinghi B.; Parente E. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204: 1849–1861; 2007.
Bettelli E.; Carrier Y.; Gao W.; Korn T.; Storm T.; Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–8; 2006.
Burgler S.; Mantel P. Y.; Bassin C.; Ouaked N.; Akdis C. A.; Schmidt-Weber C. B. RORC2 is involved in T cell polarization through interaction with the FOXP3 promoter. J. Immunol. 184: 6161–6169; 2010.
Burgler S.; Ouaked N.; Bassin C.; Basinski T. M.; Mantel P. Y.; Siegmund K.; Meyer N.; Akdis C. A.; Schmidt-Weber C. B. Differentiation and functional analysis of human T(H)17 cells. J. Allergy Clin. Immunol. 123(588–95): 595; 2009.
Cardoso C. R.; Garlet G. P.; Crippa G. E.; Rosa A. L.; Junior W. M.; Rossi M. A.; Silva J. S. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol. Immunol. 24: 1–6; 2009.
Cosmi L.; De Palma R.; Santarlasci V.; Maggi L.; Capone M.; Frosali F.; Rodolico G. Human interleukin-17-producing cells originate from a CD161+ CD4+ T-cell precursor. J. Exp. Med. 205: 1903–1916; 2008.
Crome S. Q.; Wang A. Y.; Kang C. Y.; Levings M. K. The role of retinoic acid-related orphan receptor variant 2 and IL-17 in the development and function of human CD4+ T cells. Eur. J. Immunol. 39: 1480–1493; 2009.
Ghilardi N.; Ouyang W. Targeting the development and effector functions of TH17 cells. Semin. Immunol. 19: 383–393; 2007.
Gocke A. R.; Cravens P. D.; Ben L. H.; Hussain R. Z.; Northrop S. C.; Racke M. K.; Lovett-Racke A. E. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 178: 1341–1348; 2007.
Harrington L. E.; Hatton R. D.; Mangan P. R.; Turner H.; Murphy T. L.; Murphy K. M.; Weaver C. T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6: 1123–1132; 2005.
Hirota K.; Martin B.; Veldhoen M. Development, regulation and functional capacities of Th17 cells. Semin. Immunopathol. 32: 3–16; 2010.
Ivanov I. I.; McKenzie B. S.; Zhou L.; Tadokoro C. E.; Lepelley A.; Lafaille J. J.; Cua D. J.; Littman D. R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133; 2006.
Kenneth J. L.; Thomas D. S. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ddCt Method. Methods 25: 402–408; 2001.
Kryczek I.; Wei S.; Vatan L.; Escara-Wilke J.; Szeliga W.; Keller E. T.; Zou W. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 179: 1423–1426; 2007.
Laurence A.; Tato C. M.; Davidson T. S.; Kanno Y.; Chen Z.; Yao Z.; Blank R. B.; Meylan F.; Siegel R.; Hennighausen L.; Shevach E. M.; O'Shea J. J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26: 371–381; 2007.
Lee Y. K.; Turner H.; Maynard C. L.; Oliver J. R.; Chen D.; Elson C. O.; Weaver C. T. Late developmental plasticity in the T helper 17 lineage. Immunity. 30: 92–107; 2009.
Maggi L.; Santarlasci V.; Capone M.; Peired A.; Frosali F.; Crome S. Q.; Querci V.; Fambrini M.; Liotta F.; Levings M.; Maggi E.; Cosmi L.; Romagnani S.; Annunziato F. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 40: 2174–2181; 2010.
Manel N.; Unutmaz D.; Littman D. R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgamma. Nat. Immunol. 9: 641–49; 2008.
Mangan P. R.; Harrington L. E.; O'quinn D.; Helms W.; Bullard D.; Elson C. Transforming growth factor-ß induces development of TH17 lineage. Nature 441: 231–4; 2006.
Martin-Orozco N.; Chung Y.; Chang S. H.; Wang Y. H.; Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 39: 216–224; 2009.
McGeachy M. J.; Cua D. J. Th17 cell differentiation: the long and winding road. Immunity. 28: 445–453; 2008.
Michael W. P. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: 2002–2007; 2001.
Mus A. M.; Cornelissen F.; Asmawidjaja P. S.; van Hamburg J. P.; Boon L.; Hendriks R. W.; Lubberts E. Interleukin-23 promotes Th17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not interleukin-21, in autoimmune experimental arthritis. Arthritis Rheum. 62: 1043–1050; 2010.
Naive CD4+ T cell isolation kit II human. [130-094-131]. 2008. Miltenyi Biotec GmbH. Ref Type: Catalog.
Ouyang W.; Kolls J. K.; Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 28: 454–467; 2008.
Park H.; Li Z.; Yang X. O.; Chang S. H.; Nurieva R.; Wang Y. H.; Wang Y.; Hood L.; Zhu Z.; Tian Q.; Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6: 1133–1141; 2005.
Peck A.; Mellins E. D. Plasticity of T-cell phenotype and function: the T helper type 17 example. Immunology 129: 147–153; 2009.
Shi G.; Cox C. A.; Vistica B. P.; Tan C.; Wawrousek E. F.; Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J. Immunol. 181: 7205–7213; 2008.
Streeck H.; Cohen K. W.; Jolin J. S.; Brockman M. A.; Meier A.; Power K. A.; Waring M. T.; Alter G.; Altfeld M. Rapid ex vivo isolation and long-term culture of human Th17 cells. J. Immunol. Methods 333: 115–125; 2008.
Unutmaz D. RORC2: the master of human Th17 cell programming. Eur. J. Immunol. 39: 1452–1455; 2009.
Valmori D.; Raffin C.; Raimbaud I.; Ayyoub M. Human ROR{gamma}t + TH17 cells preferentially differentiate from naive FOXP3 + Treg in the presence of lineage-specific polarizing factors. Proc. Natl. Acad. Sci. U. S. A 107: 19402–19407; 2010.
Veldhoen M.; Hirota K.; Christensen J. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206: 43–49; 2009.
Veldhoen M.; Hirota K.; Westendorf A. The aryl hydrocarbon receptor links Th17-cell-mediated autoimmunity to invironmental toxins. Nature 453: 106–109; 2010.
Veldhoen M.; Hocking R.; Atkins C.; Locksley R.; Stockinger B. TGF-ß in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189; 2006.
Volpe E.; Servant N.; Zollinger R.; Bogiatzi S. I.; Hupe P.; Barillot E.; Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9: 650–657; 2008.
Vyakarnam A. Human CD4 Culture. In: Rowland-Jones S. L.; McMichael A. (eds) Lymphocytes: a practical approach. Oxford University Press, Oxford, pp 136–142; 2000.
Wilson N. J.; Boniface K.; Chan J. R.; McKenzie B. S.; Blumenschein W. M.; Mattson J. D.; Basham B.; Smith K.; Chen T.; Morel F.; Lecron J. C.; Kastelein R. A.; Cua D. J.; McClanahan T. K.; Bowman E. P.; de Waal M. R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8: 950–957; 2007.
Yang L.; Anderson D. E.; Baecher-Allan C.; Hastings W. D.; Bettelli E.; Oukka M.; Kuchroo V. K.; Hafler D. A. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454: 350–352; 2008a.
Yang X. O.; Pappu B. P.; Nurieva R.; Akimzhanov A.; Kang H. S.; Chung Y.; Ma L.; Shah B.; Panopoulos A. D.; Schluns K. S.; Watowich S. S.; Tian Q.; Jetten A. M.; Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 28: 29–39; 2008b.
Zhu J.; Paul W. E. CD4 T cells: fates, functions, and faults. Blood 112: 1557–1569; 2008.
Acknowledgments
The main part of this work was supplied by Isfahan University of Medical Sciences (Grant No. 187058) and the remaining was financially supported by Iran National Science Foundation (Grant No. 87040290). We wish to thanks Dr. Shahshahani and Mrs Janghorban for their excellent helps in cord blood collection, Mrs Homayouni and Mrs Esmaeili for their kindly collaborations in flow cytometry and ELISA techniques and Mr. Ghoochani for his excellent helps in data interpretation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Ganjalikhani Hakemi, M., Ghaedi, K., Andalib, A. et al. Optimization of human Th17 cell differentiation in vitro: evaluating different polarizing factors. In Vitro Cell.Dev.Biol.-Animal 47, 581–592 (2011). https://doi.org/10.1007/s11626-011-9444-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-011-9444-1