Abstract
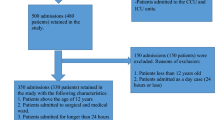
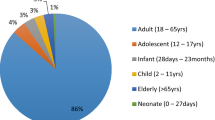
In order to improve patient safety, systematic analysis of common and repetitive patterns of preventable adverse drug reactions (pADRs) in the clinical setting should be performed regularly in order to propose adequate prevention strategies. Our aim is to evaluate the preventability of all ADRs collected in a drug information research center database, by spontaneous reporting and clinical surveillance in two internal medicine departments. One reviewer systematically reevaluated all the cases stored in the database. ADRs were deemed preventable if they were due to: a contraindication, an inadequate dose, a drug interaction, an inappropriate prescribing decision for the patient’s condition, inadequate monitoring, self-medication, or non-adherence to therapy. Out of 251 ADRs evaluated, 103 (41 %) were considered preventable. Out of the total pADRs, 86.4 % were serious. The most frequent adverse outcomes affected the gastrointestinal system (21.4 %), followed by the renal (11.6 %), metabolic (10.7 %), vascular (10.7 %) and hepatic (6.8 %) systems. Acenocoumarol (28 %), diclofenac (12.6 %), digoxin and furosemide accounted for more than 50 % of all preventable reports. One of up to three factors was involved in the preventability of the analyzed reports. Drug–drug interactions were the cause of 49.5 % of the pADRs. Inappropriate dose accounted for 17.5 % reports out of the total pADRs, inappropriate monitoring for 9.7 % reports, history of allergy to drug or drug class for 5.8 % reports and administration of a contraindicated drug for 4.8 % reports. Identifying prevalent pADRs in this study indicates a clear target for prevention strategies: drug prescription, with a special emphasis on drug interactions.
Similar content being viewed by others
References
van der Hooft CS, Sturkenboom CJM, van Groothest K, Kingma HJ, Stricker HC (2006) Adverse drug reactions-related hospitalizations. A nationwide study in The Netherlands. Drug Saf 29(2):161–168
Pouyanne P, Haramburu F, Imbs JL, Bégaud B (2000) Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. BMJ 320:1036
Pirmohamed M, James S, Meakin S et al (2004) Adverse drug reaction as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ 329:15–19
Alexpoulou A, Dourakis SY, Mantzoukis D et al (2008) Adverse drug reactions as a cause of hospital admissions: a 6-month experience in a single center in Greece. Eur J Int Med 19:505–510
Beijer HJM, Blaey CJ (2002) Hospitalizations caused by adverse drug reactions: a meta-analysis of observational studies. Pharm World Sci 24(2):46–54
Lagnaoui R, Moore N, Fach J, Longy-Boursier M, Begaud B (2000) Adverse drug reactions in a department of systemic disease-oriented internal medicine: prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol 55:181–186
Davies EC, Green CF, Mottram DR, Pirmohamed M (2006) Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther 31:335–341
Farcas A, Sinpetrean A, Mogosan C, Palage M, Vostinaru O, Bojita M, Dumitrascu D (2010) Adverse drug reactions detected by stimulated spontaneous reporting in an internal medicine department in Romania. Eur J Intern Med 21(5):453–457
Moore N, Lecointre D, Noblet C, Mabille M (1998) Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol 45:301–308
Queneau P, Bannwarth B, Carpentier F et al (2007) Emergency department visits caused by adverse drug events: results of a French survey. Drug Saf 30(1):81–88
Howard RL, Avery AJ, Howard PD, Partridge M (2003) Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care 12(4):280–285
Olivier P, Boulbes O, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2002) Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice. Drug Saf 25(14):1035–1044
Zed PJ, Abu-Laban RB, Balen RM et al (2008) Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ 178(12):1563–1569
Winterstein AG, Hatton RC, Gonzales-Rothi R, Johns TE, Segal R (2002) Identifying clinically significant preventable adverse drug events through a hospital’s database of adverse drug reactions reports. Am J Health Syst Pharm 59:1742–1749
Forster AJ, Worthington JR, Hawken S et al (2011) Using prospective clinical surveillance to identify adverse events in hospital. BMJ Qual Saf 20(9):756–763
Smits M, Zegers M, Groenewegen PP et al (2010) Exploring the causes of adverse events in hospitals and potential prevention strategies. Qual Saf Health Care 19(5):e5
Kanjanarat P, Winterstein AG, Johns TE et al (2003) Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm 60(17):1750–1759
Jönsson AK, Hakkarainen KM, Spigset O, Druid H, Hiselius A, Hägg S (2010) Preventable drug related mortality in a Swedish population. Pharmacoepidemiol Drug Saf 19(2):211–215
Hoonhout LH, de Bruijne MC, Wagner C, Asscheman H, van der Wal G, van Tulder MW (2010) Nature, occurrence and consequences of medication-related adverse events during hospitalization: a retrospective chart review in The Netherlands. Drug Saf 33(10):853–864
Brennan TA, Leape LL, Laird NM et al (1991) Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med 324(6):370–376
Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW (2004) Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care 13(4):306–314
Hug BL, Witkowski DJ, Sox CM et al (2010) Adverse drug event rates in six community hospitals and the potential impact of computerized physician order entry for prevention. J Gen Intern Med 25(1):31–38
Bates DW (1999) Frequency, consequences and prevention of adverse drug events. J Qual Clin Pract 19(1):13–17
Howard RL, Avery AJ, Slavenburg S et al (2007) Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 63(2):136–147
International Conference on Harmonisation (ICH) (1994) ICH topic E2a: clinical safety data management: definitions and standards for expedited reporting. http://www.ich.org/LOB/media/MEDIA436.pdf
Karch FE, Lasagna E (1977) Towards the operational identification of adverse drug reactions. Clin Pharmacol Ther 21(3):247–254
World Health Organization. Programme and projects: classifications [online]. http://www.who.int/classifications/icd/en/index.htm
Anatomical therapeutic chemical (ATC) classification index (1992) Geneva: WHO Collaborating Center for Drug Statistics Methodology
Micromedex® Healthcare Series [Internet database]. Greenwood Village, Thomson Reuters (Healthcare) Inc., Colo (Updated periodically)
Schumock GT, Thornton JP (1992) Focusing on the preventability of adverse drug reactions. Hosp Pharm 27(6):538
Imbs JL, Pletan Y, Spriet A (1998) Evaluation de la iatrogénèse médicamenteuse évitable: méthodologie. Therapie 53:365–370
Ducharme MM, Boothby LA (2007) Analysis of adverse drug reactions for preventability. Int J Clin Pract 61(1):157–161
Jonville-Béra AP, Béra F, Autret-Leca E (2005) Are incorrectly used drugs more frequently involved in adverse drug reactions? A prospective study. Eur J Clin Pharmacol 61(3):231–236
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2009) Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department. Drugs Aging 26(6):475–482
Becker ML, Kallewaard M, Caspers PW et al (2007) Hospitalisations and emergency department visits due to drug–drug interactions: a literature review. Pharmacoepidemiol Drug Saf 16:641–651
Bartlett G, Blais R, Tamblyn R, Clermont RJ, MacGibbon B (2008) Impact of patient communication problems on the risk of preventable adverse events in acute care settings. CMAJ 178(12):1555–1562
Acknowledgments
This study was supported by a research grant financed by the Romanian Ministry of Education, Research and Innovation—PNII 12-102/2008.
Conflict of interest
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farcas, A., Bucsa, C., Sinpetrean, A. et al. Preventability analysis of adverse drug reactions detected in two internal medicine departments in Romania. Intern Emerg Med 9, 187–193 (2014). https://doi.org/10.1007/s11739-012-0843-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-012-0843-4