Abstract
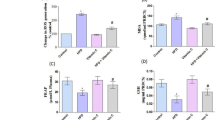
Studies have shown that non-alcoholic fatty liver disease (NAFLD) patients are more prone to cardiovascular disease (CVD). Zinc and selenium deficiency are common in NAFLD. But the effects of zinc and selenium co-supplementation before and/or after disease progression on CVD markers are not clear in NAFLD patients. This study aimed to compare the effects of zinc and selenium co-supplementation before and/or after disease progression on some of the CVD markers in an experimental model of NAFLD. Forty male Sprague Dawley rats (197 ± 4 g) were randomly assigned into four dietary groups: control group (C; received 9% of calorie as fat), model group (M; received 82% of calorie as fat), and supplementation before (BS) or after (AS) disease progression. Animals were fed diets for 20 weeks in all groups. Fasting plasma glucose (FPG), insulin, HOMA-IR, ALT, AST, lipid profile, malondialdehyde (MDA) and vascular endothelial growth factor (VEGF) levels were measured as CVD indices. Serum ALT, AST, FPG, insulin, MDA, VEGF and HOMA-IR were significantly higher in the M than C group. Co-supplementation reduced serum ALT and AST levels in the BS and AS groups compared with the M group. FPG, insulin, HOMA-IR, VEGF, MDA, LDL/HDL-c and TC/HDL-c ratio were significantly reduced in the AS compared with the M group. TG/HDL-c ratio was significantly reduced in the BS and AS compared with the M group. Serum MDA, VEGF, Insulin and HOMA-IR were significantly lowered in the AS than BS group (p < 0.05). Zinc and selenium co-supplementation after NAFLD progression reduced CVD risk indices in an experimental model.


Similar content being viewed by others
References
Ragab SM, Elghaffar SKA, El-Metwally TH, Badr G, Mahmoud MH, Omar HM (2015) Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: the ameliorative role of three natural compounds. Lipids Health Dis 14:1
Blackett PR, Sanghera DK (2013) Genetic determinants of cardiometabolic risk: a proposed model for phenotype association and interaction. Journal of clinical lipidology 7:65–81
Cakır E, Ozbek M, Colak N, Cakal E, Delıbaşi T (2012) Is NAFLD an independent risk factor for increased IMT in T2DM? Minerva Endocrinol 37:187–193
Akın L, Kurtoglu S, Yikilmaz A, Kendirci M, Elmalı F, Mazicioglu M (2013) Fatty liver is a good indicator of subclinical atherosclerosis risk in obese children and adolescents regardless of liver enzyme elevation. Acta Paediatr 102:e107–ee13
Hashizume H, Sato K, Yamazaki Y, Horiguchi N, Kakizaki S, Mori M (2013) A prospective study of long-term outcomes in female patients with nonalcoholic steatohepatitis using age-and body mass index-matched cohorts. Acta Med Okayama 67:45–53
Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ et al (2009) Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology 50:1241–1250
Murakami Y, Koyabu T, Kawashima A, Kakibuchi N, Kawakami T, Takaguchi K et al (2007) Zinc supplementation prevents the increase of transaminase in chronic hepatitis C patients during combination therapy with Pegylated interferon ALPHA-2b and ribavirin. J Nutr Sci Vitaminol 53:213–218
Takahashi M, Saito H, Higashimoto M, Hibi T (2007) Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: a pilot study. Hepatol Res 37:405–409
Matsuoka S, Matsumura H, Nakamura H, Oshiro S, Arakawa Y, Hayashi J et al (2009) Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr 45:292–303
Himoto T, Yoneyama H, Kurokohchi K, Inukai M, Masugata H, Goda F et al (2011) Selenium deficiency is associated with insulin resistance in patients with hepatitis C virus–related chronic liver disease. Nutr Res 31:829–835
do Nascimento Marreiro D, Fisberg M, Cozzolino SMF (2004) Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res 100:137–149
Tungtrongchitr R, Pongpaew P, Phonrat B, Tungtrongchitr A, Viroonudomphol D, Vudhivai N et al (2003) Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. Journal of the Medical Association of Thailand=Chotmaihet thangphaet 86:543–551
Afridi HI, Kazi TG, Kazi N, Baig JA, Jamali MK, Arain MB et al (2009) Status of essential trace metals in biological samples of diabetic mother and their neonates. Arch Gynecol Obstet 280:415–423
Viktorínová A, Tošerová E, Križko M, Ďuračková Z (2009) Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism 58:1477–1482
Farvid MS, Siassi F, Jalali M, Hosseini M, Saadat N (2004) The impact of vitamin and/or mineral supplementation on lipid profiles in type 2 diabetes. Diabetes Res Clin Pract 65:21–28
Kadhim HM, Ismail SH, Hussein KI, Bakir IH, Sahib AS, Khalaf BH et al (2006) Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J Pineal Res 41:189–193
Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J et al (2010) Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord 8:505–510
Wiernsperger N, Rapin J (2010) Trace elements in glucometabolic disorders: an update. Diabetol Metab Syndr 2:1–9
Takahashi Y, Soejima Y, Fukusato T (2012) Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 18:2300–2308
Wang X, Li H, Fan Z, Liu Y (2012) Effect of zinc supplementation on type 2 diabetes parameters and liver metallothionein expressions in Wistar rats. J Physiol Biochem 68:563–572
Da Rocha JT, Sperança A, Nogueira CW, Zeni G (2009) Hypolipidaemic activity of orally administered diphenyl diselenide in triton WR-1339-induced hyperlipidaemia in mice. J Pharm Pharmacol 61:1673–1679
Petts G, Lloyd K, Goldin R (2014) Fatty liver disease. Diagnostic Histopathology 20:102–108
Musso G, Gambino R, Cassader M (2009) Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res 48:1–26
Liu H, Lu H-y (2014) Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol 20:8407–8415
Heuer M, Kaiser GM, Kahraman A, Banysch M, Saner FH, Mathé Z et al (2012) Liver transplantation in nonalcoholic steatohepatitis is associated with high mortality and post-transplant complications: a single-center experience. Digestion 86:107–113
Bhatia LS, Curzen NP, Byrne CD (2012) Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol 27:420–428
Gaweł S, Wardas M, Niedworok E, Wardas P (2003) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci lekarskie (Warsaw, Poland: 1960) 57:453–455
Shidfar F, Jazayeri S, Mousavi SN, Malek M, Hosseini FA, Khoshpey B (2015) Does supplementation with royal jelly improve oxidative stress and insulin resistance in type 2 diabetic patients? Iranian journal of public health 44:797
Mahboob M, Rahman M, Grover P (2005) Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singap Med J 46:322
Valfrè di Bonzo L, Novo E, Cannito S, Busletta C, Paternostro C, Povero D, et al (2009) Angiogenesis and liver fibrogenesis
Sanz-Cameno P, Trapero-Marugán M, Chaparro M, Jones EA, Moreno-Otero R (2010) Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. Journal of oncology 2010
Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J (2009) Angiogenesis in liver disease. J Hepatol 50:604–620
Kukla M, Gabriel A, Sabat D, Liszka Ł, Wilk M, Petelenz M et al (2010) Association between liver steatosis and angiogenesis in chronic hepatitis C. Pol J Pathol 61:154–160
Ciupińska-Kajor M, Hartleb M, Kajor M, Kukla M, Wyleżoł M, Lange D et al (2013) Hepatic angiogenesis and fibrosis are common features in morbidly obese patients. Hepatol Int 7:233–240
Rosmorduc O, Housset C, editors (2010). Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Seminars in liver disease: © Thieme Medical Publishers.
Kukla M (2013) Angiogenesis: a phenomenon which aggravates chronic liver disease progression. Hepatol Int 7:4–12
Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H (2011) Angiogenesis in chronic liver disease and its complications. Liver Int 31:146–162
Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F et al (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364:937–952
Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF et al (2009) Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 5:757
Kim J, Lee S (2012) Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women. Nutrition research and practice 6:221–225
Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X et al (2014) High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol Lett 224:16–23
Tagaram HRS, Desai D, Li G, Liu D, Rountree CB, Gowda K et al (2016) A selenium containing inhibitor for the treatment of hepatocellular cancer. Pharmaceuticals 9:18
Dong H, Yuan N, Sun T, Dun A, Hou H (2016) Effects of selenium supplement on atherogenesis of ApoE-knockout mice fed high fat diet. Zhonghua xin xue guan bing za zhi 44:244–249
Nardinocchi L, Pantisano V, Puca R, Porru M, Aiello A, Grasselli A, Leonetti C, Safran M, Rechavi G, Givol D, Farsetti A, D'Orazi G (2010) Zinc downregulates HIF-1α and inhibits its activity in tumor cells in vitro and in vivo. PLoS One 12:e15048
Golovine K, Uzzo GR, Makhov P, Crispen LP, Kunkle D, Kolenko MV (2008) Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL- 6 and IL- 8 in prostate cancer cells via NF-kB-dependent pathway. Prostate 68:1443–1449
Steinbrenner H, Sies H (2009) Protection against reactive oxygen species by selenoproteins. Biochimica et Biophysica Acta (BBA)-General Subjects 1790:1478–1485
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727
Ozkaya M, Sahin M, Cakal E, Gisi K, Bilge F, Kilinc M (2009) Selenium levels in first-degree relatives of diabetic patients. Biol Trace Elem Res 128:144–151
Wijesekara N, Chimienti F, Wheeler M (2009) Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab 11:202–214
Prasad AS (2008) Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol 43:370–377
Wiernsperger N (2003) Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes & metabolism 29:579–585
Suliburska J, Bogdański P, Pupek-Musialik D, Krejpcio Z (2011) Dietary intake and serum and hair concentrations of minerals and their relationship with serum lipids and glucose levels in hypertensive and obese patients with insulin resistance. Biol Trace Elem Res 139:137–150
Singh RB, Niaz MA, Rastogi SS, Bajaj S, Gaoli Z, Shoumin Z (1998) Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr 17:564–570
Soinio M, Marniemi J, Laakso M, Pyörälä K, Lehto S, Rönnemaa T (2007) Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 30:523–528
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Mousavi, S.N., Faghihi, A., Motaghinejad, M. et al. Zinc and Selenium Co-supplementation Reduces Some Lipid Peroxidation and Angiogenesis Markers in a Rat Model of NAFLD-Fed High Fat Diet. Biol Trace Elem Res 181, 288–295 (2018). https://doi.org/10.1007/s12011-017-1059-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1059-2