Abstract
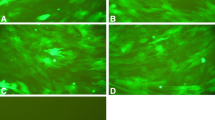
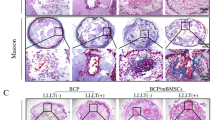
To investigate the functions of triple point-mutants of hypoxia-inducible factor 1α (HIF1α) in angiogenesis in bone defect regions under normoxic conditions. 1. Triple point-mutations (in amino acids 402, 564, and 803) in the HIF1α coding sequence (CDS) were induced by polymerase chain reaction. The triple mutant HIF1α (402/564/803) was inserted into the adenovirus pAdEasy-1 system for complete viral packaging and titer measurements. 2. For the in vitro experiment, rabbit bone marrow mesenchymal stem cells (MSCs) were divided into four experimental groups. The efficiency of infection was observed by the expression of human renilla reniformis green fluorescent protein (hrGFP). The HIF1α mRNA, protein and VEGF protein expression levels in infected cells in each experimental group were measured. 3. As in the in vivo experiment, the MSCs were divided into four groups and infected with the viral solutions from each complementary in vitro group and cultured under normoxic conditions. The MSCs were used as seed cells and transplanted into an apatite–wollastonite magnetic bioactive glass–ceramic (AW MGC) vector to construct artificial tissue-engineering scaffolds that were then implanted into the in vivo rabbit radial bone defect model. The animals from each group were killed 8 weeks after the surgery, and the tissues from the implantation region were harvested for the evaluation of the angiogenesis. 1. The 402,564, and 803 amino acids in CDS area were point mutated into alanine; three types of recombinant adenovirus were successfully constructed, packaged, and characterized. 2. The expression levels of HIF1α mRNA in A and B groups were significantly higher than those in the C and D groups (P < 0.05). The HIF1α and VEGF protein expression levels in A group were significantly higher than those in the other three groups (P < 0.05). 3. There was prominent angiogenesis in bone defect regions in group A animals. 1. Triple point-mutants of HIF1α efficiently expressed functional proteins under normoxic conditions. 2. Triple point-mutants HIF1α effectively promoted in vivo angiogenesis in bone defect regions.









Similar content being viewed by others
References
Lu, C. Y., Marcucio, R., & Miclau, T. (2006). Assessing angiogenesis during fracture healing. Iowa Orthopaedic Journal, 26, 17–26.
Seregin, S. S., & Amalfitano, A. (2009). Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opinion on Biological Therapy, 9, 1521–1531.
Fiorenzo, P., Mongiardi, M. P., Dimitri, D., et al. (2010). HIF1-positive and HIF1-negative glioblastoma cells compete in vitro but cooperate in tumor growth in vivo. International Journal of Oncology, 36, 785–791.
Giatromanolaki, A., Fiska, A., Pitsiava, D., et al. (2009). Erythropoietin receptors in endometrial carcinoma as related to HIF1{alpha} and VEGF expression. In Vivo, 23, 699–703.
Semenza, G. L. (2007). Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discovery Today, 12, 853–859.
Wan, C., Gilbert, S. R., Wang, Y., et al. (2008). Activation of the hypoxia-inducible factor-1-alpha pathway accelerates. Proceedings of the National Academy of Sciences, 105, 686–691.
Wang, Y., Wan, C., Deng, L., et al. (2007). The hypoxia-inducible factor 1 alpha pathway couples angiogenesis to osteogenesis during skeletal development. The Journal of Clinical Investigation, 117, 1616–1626.
Khan, T. A., Sellke, F. W., & Laham, R. J. (2003). Gene therapy progress and prospects: therapeutic angiogenesis for limb and myocardial ischemia. Gene Therapy, 10, 285–291.
Giaccia, A., Siim, B. G., & Johnson, R. S. (2003). HIF-1 as a target for drug development. Nature Reviews Drug Discovery, 2, 803–811.
Ceradini, D. J., Yao, D., Grogan, R. H., et al. (2008). Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. Journal of Biological Chemistry, 283, 10930–10938.
Evans, C. E., Humphries, J., Mattock, K., et al. (2010). Hypoxia and upregulation of hypoxia- inducible factor 1{alpha} stimulate venous thrombus recanalization. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 2443–2451.
Kazi, A. A., Molitoris, K. H., & Koos, R. D. (2009). Estrogen rapidly activates the PI3 K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biology of Reproduction, 81, 378–387.
Bruick, R. K., & McKnight, S. L. (2001). A conserved family of prolyl-4-hydroxylases that modify HIF. Science, 294, 1337–1340.
Szuromi, P. (2001). The enzymology of oxygen sensing. Science, 294, 1239.
Lando, D., Peet, D. J., Whelan, D. A., et al. (2002). Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science, 295, 858–861.
Lim, W., Cho, J., Kwon, H. Y., et al. (2009). Hypoxia-inducible factor 1 alpha activates and is inhibited by unoccupied estrogen receptor beta. FEBS Letters, 583, 1314–1318.
Emans, P. J., Spaapen, F., Surtel, D. A., et al. (2007). A novel in vivo model to study endochondral bone formation; HIF-1α activation and BMP expression. Bone, 40, 409–418.
Simon, F., Bockhorn, M., Praha, C., et al. (2010). Deregulation of HIF1-alpha and hypoxia- regulated pathways in hepatocellular carcinoma and corresponding non-malignant liver tissue–influence of a modulated host stroma on the prognosis of HCC. Langenbeck’s Archives of Surgery, 395, 395–405.
Elson, D. A., Thurston, G., Huang, L. E., et al. (2001). Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes & Development, 15, 2520–2532.
Acknowledgments
This project is funded by the Liaoning Affiliated Hospital Youth Science and Technology Startup Fund (FY2012-04).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, C., Liu, D., Zhang, Z. et al. Triple Point-Mutants of Hypoxia-Inducible Factor-1α Accelerate In Vivo Angiogenesis in Bone Defect Regions. Cell Biochem Biophys 67, 557–566 (2013). https://doi.org/10.1007/s12013-013-9541-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9541-8