Abstract
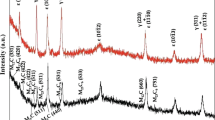
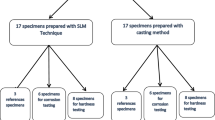
We sought to study the corrosion behavior and surface properties of a commercial cobalt–chromium (Co–Cr) alloy which was fabricated with selective laser melting (SLM) technique. For this purpose, specimens were fabricated using different techniques, such as SLM system and casting methods. Surface hardness testing, microstructure observation, surface analysis using X-ray photoelectron spectroscopy (XPS) and electrochemical corrosion test were carried out to evaluate the corrosion properties and surface properties of the specimens. We found that microstructure of SLM specimens was more homogeneous than that of cast specimens. The mean surface hardness values of SLM and cast specimens were 458.3 and 384.8, respectively; SLM specimens showed higher values than cast ones in hardness. Both specimens exhibited no differences in their electrochemical corrosion properties in the artificial saliva through potentiodynamic curves and EIS, and no significant difference via XPS. Therefore, we concluded that within the scope of this study, SLM-fabricated restorations revealed good surface properties, such as proper hardness, homogeneous microstructure, and also showed sufficient corrosion resistance which could meet the needs of dental clinics.









Similar content being viewed by others
References
Fasbinder, D. J. (2006). Clinical performance of chairside CAD/CAM restorations. Journal of the American Dental Association, 137, 21S–31S.
Yadroitsev, I., Bertrand, P. H., & Smurov, I. (2007). Parametric analysis of the selective laser melting process. Applied Surface Science, 253, 8064–8069.
Viennot, S., Dalard, F., Lissac, M., & Grosgogeat, B. (2005). Corrosion resistance of cobalt–chromium and palladium-silver alloys used in fixed prosthetic restorations. European Journal of Oral Science, 113, 90–95.
Qiu, J., Yu, W. Q., & Zhang, F. Q. (2011). Effects of the porcelain-fused-to-metal firing process on the surface and corrosion of two Co–Cr dental alloys. Journal of Material Science, 46, 1359–1368.
Qiu, J., Yu, W.-Q., Zhang, F.-Q., et al. (2011). Corrosion behavior and surface analysis of a Co–Cr and two Ni–Cr dental alloys before and after simulated porcelain firing. European Journal of Oral Science, 119, 93–101.
Viswanathan, S. S., & Choe, H. C. (2009). Electrochemical behavior of Co–Cr and Ni–Cr dental cast alloys. Trans Nonferrous Metal Society of China, 19, 785–790.
Hsu, R. W. W., Yang, C. C., Huang, C. A., & Chen, Y. S. (2005). Electrochemical corrosion studies on Co–Cr–Mo implant alloy in biological solutions. Material Chemistry and Physics, 93, 531–538.
Aparicio, C., Gil, F. J., & Fonseca, C. (2003). Corrosion behavior of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials, 24, 263–273.
Ameer, M. A., & Khamis, E. (2004). Electrochemical behaviour of recasting Ni–Cr and Co–Cr non-precious dental alloys. Corrosion Science, 46, 2825–2836.
Muñoz, A. I., & Julián, L. C. (2010). Influence of electrochemical potential on the tribocorrosion behaviour of high carbon CoCrMo biomedical alloy in simulated body fluids by electrochemical impedance spectroscopy. Electrochimica Acta, 55, 5428–5439.
Hodgson, A. W. E., Kure, S., Virtanen, S., Fervel, V., Olsson, C. O. A., & Mischler, S. (2004). Passive and trans-passive behavior of CoCrMo in simulated biological solutions. Electrochimica Acta, 49, 2167–2178.
Hanawa, T., Hiromoto, S., & Asami, K. (2001). Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Applied Surface Science, 183, 68–75.
Survilienė, S., Jasulaitienė, V., Češūnienė, A., & Oleksiak, L. (2008). The use of XPS for study of the surface layers of Cr–Co alloy electrodeposited from Cr(III) formate-urea baths. Solid State Ionics, 179, 222–227.
Kocijan, A., Milosev, I., & Pihlar, B. (2004). Cobalt-based alloys for orthopedic applications studied by electrochemical and XPS analysis. Journal of Material Science and Materials in Medicine, 15, 643–650.
Acknowledgments
This study was supported by Shanghai Leading Academic Discipline Project (Grant No. S30206), National Basic Research Program of China (Grant No. 2012CB910400) and Science and Technology Committee of Shanghai (Grant Nos. 08DZ2271100 and 12441903001). The authors thank personnel from the School of Materials Science and Engineering for their support and cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xin, Xz., Chen, J., Xiang, N. et al. Surface Properties and Corrosion Behavior of Co–Cr Alloy Fabricated with Selective Laser Melting Technique. Cell Biochem Biophys 67, 983–990 (2013). https://doi.org/10.1007/s12013-013-9593-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9593-9