Abstract
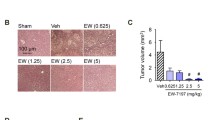
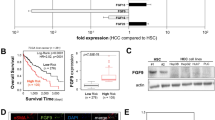
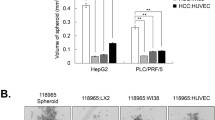
We investigated the mechanism and effects of sorafenib on hepatic stellate cell (HSC) viability and in the liver tumor microenvironment. The expression of α-smooth muscle actin (α-SMA) was measured immunocytochemically in the LX2 cells treated with differing concentrations of sorafenib. Changes in the platelet-derived growth factor (PDGF)-BB and tumor growth factor (TGF)-β1 concentrations were detected in the LX2 supernatant using an enzyme-linked immunosorbent assay (ELISA). Expressions of the extracellular signal-regulated kinase 1 (ERK1), ERK2, and Akt signaling pathways were measured using a western blot assay. The LX2 cells were cocultured with HepG2 cells for 24 h to observe their effects on HepG2 cell invasive ability. (1) After treatment with various concentrations of sorafenib for 12, 24, 36, or 48 h, MTT assay showed that the viability of the treated LX2 cells was lower than in the controls. (2) As sorafenib concentration and time of exposure increased, α-SMA expression became weaker in the treated cells. (3) The PDGF-BB and TGF-β1 concentrations decreased with higher concentration, and longer exposures under the same sorafenib concentration. (4) The ERK1, ERK2, and Akt expressions were identical between the treated and the control groups, but their phosphorylated expression decreased with increased concentrations of sorafenib. (5) The invasive ability of the HepG2 cells induced by the LX2 gradually decreased as sorafenib concentrations increased. Sorafenib suppressed α-SMA expression, inhibited PDGF-dependent signaling pathways in HSCs, downregulated the PDGF-BB and TGF-β1 expression in the HSCs supernatant, and restrained viability of the HSCs, resulting in suppressed proliferation and invasion in the HepG2 cells.






Similar content being viewed by others
References
Ferlay, J., Shin, H. R., Bray, F., Forman, D., Mathers, C., & Parkin, D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127, 2893–2917.
Yang, J. D., Nakamura, I., & Roberts, L. R. (2011). The tumor microenvironment in hepatocellular carcinoma: Current status and therapeutic targets. Seminars in Cancer Biology, 21, 35–43.
Amann, T., Bataille, F., Spruss, T., Mühlbauer, M., Gäbele, E., Schölmerich, J., et al. (2009). Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Science, 100, 646–653.
Wilhelm, S. M., Adnane, L., Newell, P., Villanueva, A., Llovet, J. M., & Lynch, M. (2008). Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Molecular Cancer Therapeutics, 7, 3129–3140.
Faouzi, S., Lepreux, S., Bedin, C., Dubuisson, L., Balabaud, C., Bioulac-Sage, P., et al. (1999). Activation of cultured rat hepatic stellate cells by tumoral hepatocytes. Laboratory Investigation, 79, 485–493.
Thabut, D., Routray, C., Lomberk, G., Shergill, U., Glaser, K., Huebert, R., et al. (2011). Complementary vascular and matrix regulatory pathways underlie the beneficial mechanism of action of sorafenib in liver fibrosis. Hepatology, 54, 573–585.
Olaso, E., Salado, C., Egilegor, E., et al. (2003). Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology, 37, 674–685.
Pinzani, M., Gesualdo, L., Sabbah, G. M., & Abboud, H. E. (1989). Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. The Journal of Clinical Investigation, 84, 1786–1793.
Yu, J., Ustach, C., & Kim, H. R. (2003). Platelet-derived growth factor signaling and human cancer. Journal of Biochemistry and Molecular Biology, 36, 49–59.
Boner, J. C. (2004). Regulation of PDGF and its receptors in fibrotic diseases. Cytokine & Growth Factor Reviews, 15, 255–273.
Gressner, A. M., Weiskirchen, R., Breitkopf, K., & Dooley, S. (2002). Roles of TGF-beta in hepatic fibrosis. Frontiers in Bioscience, 7, d793–d807.
Rasanen, K., & Vaheri, A. (2010). Activation of fibroblasts in cancer stroma. Experimental Cell Research, 316(17), 2713–2722.
Pinzani, M. (2002). PDGF and signal transduction in hepatic stellate cells. Frontiers in Bioscience, 7, d1720–d1726.
Marra, F., Pinzani, M., DeFranco, R., Laffi, G., & Gentilini, P. (1995). Involvement of phosphatidylinositol 3-kinase in the activation of extracellular signal-regulated kinase by PDGF in hepatic stellate cells. FEBS Letters, 376, 141–145.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30971340) and CSCO–Bayer Schering Liver Cancer Fund for Young Physicians (2010).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Geng, Zm., Jha, R.K., Li, B. et al. Sorafenib Inhibition of Hepatic Stellate Cell Proliferation in Tumor Microenvironment of Hepatocellular Carcinoma: A Study of the Sorafenib Mechanisms. Cell Biochem Biophys 69, 717–724 (2014). https://doi.org/10.1007/s12013-014-9858-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9858-y