Abstract
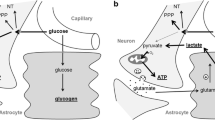
Microdialysis, an in vivo technique that permits collection and analysis of small molecular weight substances from the interstitial space, was developed more than 30 years ago and introduced into the clinical neurosciences in the 1990s. Today cerebral microdialysis is an established, commercially available clinical tool that is focused primarily on markers of cerebral energy metabolism (glucose, lactate, and pyruvate) and cell damage (glycerol), and neurotransmitters (glutamate). Although the brain comprises only 2% of body weight, it consumes 20% of total body energy. Consequently, the ability to monitor cerebral metabolism can provide significant insights during clinical care. Measurements of lactate, pyruvate, and glucose give information about the comparative contributions of aerobic and anaerobic metabolisms to brain energy. The lactate/pyruvate ratio reflects cytoplasmic redox state and thus provides information about tissue oxygenation. An elevated lactate pyruvate ratio (>40) frequently is interpreted as a sign of cerebral hypoxia or ischemia. However, several other factors may contribute to an elevated LPR. This article reviews potential non-hypoxic/ischemic causes of an increased LPR.





Similar content being viewed by others
Abbreviations
- CBF:
-
Cerebral blood flow
- CMD:
-
Cerebral microdialysis
- ETC:
-
Electron transport chain
- G6P:
-
Glucose-6-phosphate
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- ICP:
-
Intracranial pressure
- LDH:
-
Lactate dehydrogenase
- LPR:
-
Lactate/pyruvate ratio
- LPS:
-
Lipopolysaccharide
- MAS:
-
Malate-aspartate shuttle
- NMR:
-
Nuclear magnetic resonance
- PDH:
-
Pyruvate dehydrogenase
- PG:
-
Propylene glycol
- SAH:
-
Subarachnoid hemorrhage
- TBI:
-
Traumatic brain injury
References
Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweiz Akad Med Wiss. 1974;30:44–55.
Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009;47:7.1.1–1.28.
Torregrossa MM, Kalivas PW. Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav. 2008;90:261–72.
Meeusen R. Exercise and the brain: insight in new therapeutic modalities. Ann Transplant. 2005;10:49–51.
van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90:135–47.
Li Y, Peris J, Zhong L, Derendorf H. Microdialysis as a tool in local pharmacodynamics. AAPS J. 2006;8:E222–35.
Stiller CO, Taylor BK, Linderoth B, Gustafsson H, Warsame Afrah A, Brodin E. Microdialysis in pain research. Adv Drug Deliv Rev. 2003;55:1065–79.
Meyerson BA, Linderoth B, Karlsson H, Ungerstedt U. Extracellular measurements in the thalamus of parkinsonian patients. Life Sci. 1990;46:301–8.
Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22:3–41.
Bellander BM, Cantais E, Enblad P, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–9.
Belli A, Sen J, Petzold A, Russo S, Kitchen N, Smith M. Metabolic failure precedes intracranial pressure rises in traumatic brain injury: a microdialysis study. Acta Neurochir (Wien). 2008;150:461–9.
Adamides AA, Rosenfeldt FL, Winter CD, et al. Brain tissue lactate elevations predict episodes of intracranial hypertension in patients with traumatic brain injury. J Am Coll Surg. 2009;209:531–9.
Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Curr Pharm Des. 2004;10:2145–52.
Goodman JC, Robertson CS. Microdialysis: is it ready for prime time? Curr Opin Crit Care. 2009;15:110–7.
Nordström CH. Cerebral energy metabolism and microdialysis in neurocritical care. Childs Nerv Syst. 2010;26:465–72.
Tisdall MM, Smith M. Cerebral microdialysis: research technique or clinical tool. Br J Anaesth. 2006;97:18–25.
Oddo M, Schmidt JM, Carrera E, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36:3233–8.
Vespa PM, McArthur D, O’Phelan K, et al. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab. 2003;23:865–77.
Oddo M, Milby A, Chen I, et al. Hemoglobin concentration and cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2009;40:1275–81.
Nordström CH, Reinstrup P, Xu W, Gärdenfors A, Ungerstedt U. Assessment of the lower limit for cerebral perfusion pressure in severe head injuries by bedside monitoring of regional energy metabolism. Anesthesiology. 2003;98:809–14.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6.
Hashemi P, Bhatia R, Nakamura H, et al. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leão’s spreading depression. J Cereb Blood Flow Metab. 2009;29:166–75.
Schneweis S, Grond M, Staub F, et al. Predictive value of neurochemical monitoring in large middle cerebral artery infarction. Stroke. 2001;32:1863–7.
Nortje J, Coles JP, Timofeev I, et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit Care Med. 2008;36:273–81.
Marcoux J, McArthur DA, Miller C, et al. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. 2008;36:2871–7.
Hillered L, Persson L, Nilsson P, Ronne-Engstrom E, Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr Opin Crit Care. 2006;12:112–8.
Leegsma-Vogt G, van der Werf S, Venema K, Korf J. Modeling cerebral arteriovenous lactate kinetics after intravenous lactate infusion in the rat. J Cereb Blood Flow Metab. 2004;24:1071–80.
Miller LP, Oldendorf WH. Regional kinetic constants for blood-brain barrier pyruvic acid transport in conscious rats by the monocarboxylic acid carrier. J Neurochem. 1986;46:1412–6.
Hutchinson PJ, O’Connell MT, Al-Rawi PG, et al. Clinical cerebral microdialysis: a methodological study. J Neurosurg. 2000;93:37–43.
Nelson DL, Cox MM. Lehninger principles of biochemistry. 5th ed. New York: WH Freeman; 2008.
Johnston AJ, Gupta AK. Advanced monitoring in the neurology intensive care unit: microdialysis. Curr Opin Crit Care. 2002;8:121–7.
Reinstrup P, Ståhl N, Mellergård P, Uski T, Ungerstedt U, Nordström CH. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47:701–9.
Gårdenfors A, Nilsson F, Skagerberg G, Ungerstedt U, Nordström CH. Cerebral physiological and biochemical changes during vasogenic brain edema induced by intrathecal injection of bacterial lipopolysaccharides in piglets. Acta Neurochir. 2002;144:601–8.
Dusick JR, Glenn TC, Lee WN, et al. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1, 2–13C2]glucose labeling study in humans. J Cereb Blood Flow Metab. 2007;27:1593–602.
Vespa P, Bergsneider M, Hattori N, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–4.
Simpson NE, Han Z, Berendzen KM, et al. Magnetic resonance spectroscopic investigation of mitochondrial fuel metabolism and energetics in cultured human fibroblasts: effects of pyruvate dehydrogenase complex deficiency and dichloroacetate. Mol Genet Metab. 2006;89:97–105.
Nakasaki H, Ohta M, Soeda J, et al. Clinical and biochemical aspects of thiamine treatment for metabolic acidosis during total parenteral nutrition. Nutrition. 1997;13:110–7.
Tinsa F, Ben Amor S, Kaabachi N, Ben Lasouad M, Boussetta K, Bousnina S. Unusual case of thiamine responsive megaloblastic anemia. Tunis Med. 2009;87:159–63.
Poggi-Travert F, Martin D, Billette de Villemeur T, et al. Metabolic intermediates in lactic acidosis: compounds, samples and interpretation. J Inherit Metab Dis. 1996;19:478–88.
Stacpoole PW, Wright EC, Baumgartner TG, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. NEJM. 1992;327:1564–9.
Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80:71–7.
Moreadith RW, Batshaw ML, Ohnishi T, et al. Deficiency of the iron-sulfur clusters of mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase (complex I) in an infant with congenital lactic acidosis. J Clin Invest. 1984;74:685–97.
Robinson BH, McKay N, Goodyer P, Lancaster G. Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacitcacidemia. Am J Hum Genet. 1985;37:938–46.
Van Hove JL, Saenz MS, Thomas JA, et al. Succinyl-CoA ligase deficiency: a mitochondrial hepatoencephalomyopathy. Pediatr Res. 2010;68:159–64.
Chesney RW, Kaplan BS, Colle E, et al. Abnormalities of carbohydrate metabolism in idiopathic Fanconi syndrome. Pediatr Res. 1980;14:209–15.
Boustany RN, Aprille JR, Halperin J, Levy H, DeLong GR. Mitochondrial cytochrome deficiency presenting as a myopathy with hypotonia, external ophthalmoplegia, and lactic acidosis in an infant and as fatal hepatopathy in a second cousin. Ann Neurol. 1983;14:462–70.
Komaki H, Nishigaki Y, Fuku N, et al. Pyruvate therapy for Leigh syndrome due to cytochrome c oxidase deficiency. Biochim Biophys Acta. 2010;1800:313–5.
Mannan AA, Sharma MC, Shrivastava P, et al. Leigh’s syndrome. Indian J Pediatr. 2004;71:1029–33.
Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis. 2007;22:199–218.
Lin S, Raabe W. Ammonia intoxication: effects on cerebral cortex and spinal cord. J Neurochem. 1985;44:1252–8.
Adams JM, Feustel PJ, Donnelly DF, Dutton RE. Hypoxia, hyperammonemia, and cerebrospinal fluid metabolites. Adv Shock Res. 1978;1:209–20.
O’Connor JE, Costell M, Grisolía S. Prevention of ammonia toxicity by L-carnitine: metabolic changes in brain. Neurochem Res. 1984;9:563–70.
Hindfelt B, Plum F, Duffy TE. Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest. 1977;59:386–96.
Bjerring PN, Hauerberg J, Frederiksen HJ, et al. Cerebral glutamine concentration and lactate-pyruvate ratio in patients with acute liver failure. Neurocrit Care. 2008;9:3–7.
Ratnakumari L, Murthy CR. Response of rat cerebral glycolytic enzymes to hyperammonemic states. Neurosci Lett. 1993;161:37–40.
Qureshi K, Rama Rao KV, Qureshi IA. Differential inhibition by hyperammonemia of the electron transport chain enzymes in synaptosomes and non-synaptic mitochondria in ornithine transcarbamylase-deficient spf-mice: restoration by acetyl-L-carnitine. Neurochem Res. 1998;23:855–61.
Ratnakumari L, Qureshi IA, Butterworth RF. Effects of congenital hyperammonemia on the cerebral and hepatic levels of the intermediates of energy metabolism in spf mice. Biochem Biophys Res Commun. 1992;184:746–51.
She P, Zhou Y, Zhang Z, Griffin K, Gowda K, Lynch CJ. Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance. J Appl Physiol. 2010;108:941–9.
Kauppinen RA, Sihra TS, Nicholls DG. Aminooxyacetic acid inhibits the malate-aspartate shuttle in isolated nerve terminals and prevents the mitochondria from utilizing glycolytic substrates. Biochim Biophys Acta. 1987;930:173–8.
Nagasaka H, Okano Y, Tsukahara H, et al. Sustaining hypercitrullinemia, hypercholesterolemia and augmented oxidative stress in Japanese children with aspartate/glutamate carrier isoform 2-citrin-deficiency even during the silent period. Mol Genet Metab. 2009;97:21–6.
Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–93.
Michenfelder JD, Theye RA. In vivo toxic effects of halothane on canine cerebral metabolic pathways. Am J Physiol. 1975;229:1050–5.
Dahlbacka S, Mäkelä J, Kaakinen T, et al. Propofol is associated with impaired brain metabolism during hypothermic circulatory arrest: an experimental microdialysis study. Heart Surg Forum. 2006;9:E710–8.
Carles M, Dellamonica J, Roux J, et al. Sevoflurane but not propofol increases interstitial glycolysis metabolites availability during tourniquet-induced ischaemia-reperfusion. Br J Anaesth. 2008;100:29–35.
Marian M, Parrino C, Leo AM, Vincenti E, Bindoli A, Scutari G. Effect of the intravenous anesthetic 2,6-diisopropylphenol on respiration and energy production by rat brain synaptosomes. Neurochem Res. 1997;22:287–92.
Branca D, Roberti MS, Lorenzin P, Vincenti E, Scutari G. Influence of the anesthetic 2,6-diisopropylphenol on the oxidative phosphorylation of isolated rat liver mitochondria. Biochem Pharmacol. 1991;42:87–90.
Fudickar A, Bein B. Propofol infusion syndrome: update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009;75:339–44.
Pisapia JM, Wendell LC, Kumar MA, Zager EL, Levine JM. Lactate-to-pyruvate ratio as a marker of propofol infusion syndrome after subarachnoid hemorrhage. Neurocrit Care. 2010. doi:10.1007/s12028-010-9467-6.
Nam YT, Kim JS, Park KW. Effects of hypotensive anesthesia with sodium nitroprusside or isoflurane on hemodynamic and metabolic changes. Yonsei Med J. 1992;33:320–5.
Michenfelder JD. Cyanide release from sodium nitroprusside in the dog. Anesthesiology. 1977;46:196–201.
Tinker JH, Michenfelder JD. Cardiac cyanide toxicity induced by nitroprusside in the dog: potential for reversal. Anesthesiology. 1978;49:109–16.
Dai YL, Luk TH, Siu CW, et al. Mitochondrial dysfunction induced by statin contributes to endothelial dysfunction in patients with coronary artery disease. Cardiovasc Toxicol. 2010;10:130–8.
Ruddick JA. Toxicology, metabolism, and biochemistry of 1,2-propanediol. Toxicol Appl Pharmacol. 1972;21:102–11.
Saini M, Nagpaul JP, Amma MK. Effect of propane-1,2-diol ingestion on carbohydrate metabolism in female rat erythrocytes. J Appl Toxicol. 1993;13:69–75.
Morshed KM, Nagpaul JP, Majumdar S, Amma MKP. Kinetics of oral propylene glycol-induced acute hyperlactatemia. Biochem Med Metab Biol. 1989;42:87–94.
Schölmerich J, Kitamura S, Miyai K. Effects of propylene glycol on redox state of the perfused rat liver—a note of caution. Res Exp Med (Berl). 1989;189:39–42.
Wilson KC, Reardon C, Theodore AC, Farber HW. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines. Chest. 2005;128:1674–81.
Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy. 2006;26:23–33.
Ganesh A, Audu P. Hyperosmolar, increased-anion-gap metabolic acidosis and hyperglycemia after etomidate infusion. J Clin Anesth. 2008;20:290–3.
Szajewski J. Poisons information monographs: Propylene glycol. No. 443. Geneva, Switzerland: International Programme on Chemical Safety, 1994.
Zar T, Graeber C, Perazella MA. Recognition, treatment, and prevention of propylene glycol toxicity. Sem Dial. 2007;20:217–9.
Rengel-Aranda M, Gougoux A, Vinay P, Lopez-Novoa JM. Effect of valproate on renal metabolism in the intact dog. Kidney Int. 1988;34:645–54.
Dubas TC, Johnson WJ. Metformin-induced lactic acidosis: potentiation by ethanol. Res Commun Chem Pathol Pharmacol. 1981;33:21–31.
Jalling O, Olsen C. The effects of metformin compared to the effects of phenformin on the lactate production and the metabolism of isolated parenchymal rat liver cell. Acta Pharmacol Toxicol (Copenh). 1984;54:327–32.
Nattrass M, Hinks L, Smythe P, Todd PG, Alberti KGMM. Metabolic effects of combined sulphonylurea and metformin therapy in maturity-onset diabetes. Horm Metab Res. 1979;11:332–7.
Ramanathan S, Masih AK, Ashok U, Arismendy J, Turndorf H. Concentrations of lactate and pyruvate in maternal and neonatal blood with different intravenous fluids used for prehydration before epidural anesthesia. Anesth Analg. 1984;63:69–74.
Pellerin L, Bouzier-Sore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–62.
Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–71.
Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16:1079–89.
Abi-Saab WM, Maggs DG, Jones T, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22:271–9.
Agardh CD, Folbergrová J, Siesjö BK. Cerebral metabolic changes in profound, insulin-induced hypoglycemia, and in the recovery period following glucose administration. J Neurochem. 1978;31:1135–42.
Cardell M, Siesjö BK, Wieloch T. Changes in pyruvate dehydrogenase complex activity during and following severe insulin-induced hypoglycemia. J Cereb Blood Flow Metab. 1991;11:122–8.
Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;34:1200–7.
Vespa P, Boonyaputthikul R, McArthur DL, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–6.
Kennan RP, Takahashi K, Pan C, Shamoon H, Pan JW. Human cerebral blood flow and metabolism in acute insulin-induced hypoglycemia. J Cereb Blood Flow Metab. 2005;25:527–34.
Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–63.
Eckert B, Ryding E, Agardh CD. Sustained elevation of cerebral blood flow after hypoglycaemia in normal man. Diabetes Res Clin Pract. 1998;40:91–100.
Rosdahl H, Samuelsson AC, Ungerstedt U, Henriksson J. Influence of adrenergic agonists on the release of amino acids from rat skeletal muscle studied by microdialysis. Acta Physiol Scand. 1998;163:349–60.
Levy B, Mansart A, Bollaert PE, Franck P, Mallie JP. Effects of epinephrine and norepinephrine on hemodynamics, oxidative metabolism, and organ energetics in endotoxemic rats. Intensive Care Med. 2003;29:292–300.
Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med. 1997;23:282–7.
Pernet A, Walker M, Gill GV, Ørskov H, Alberti KGMM, Johnston DG. Metabolic effects of adrenaline and noradrenaline in man: studies with somatostatin. Diabete Metab. 1984;10:98–105.
Christensen NJ, Alberti KG, Brandsborg O. Plasma catecholamines and blood substrate concentrations: studies in insulin induced hypoglycaemia and after adrenaline infusions. Eur J Clin Invest. 1975;5:415–23.
Heringlake M, Wernerus M, Grünefeld J, et al. The metabolic and renal effects of adrenaline and milrinone in patients with myocardial dysfunction after coronary artery bypass grafting. Crit Care. 2007;11:R51.
Cano A, Martínez P, Parrilla JJ, Abad L. Effects of intravenous ritodrine on lactate and pyruvate levels: role of glycemia and anaerobiosis. Obstet Gynecol. 1985;66:207–10.
d’Avila JC, Santiago AP, Amâncio RT, et al. Sepsis induces brain mitochondrial dysfunction. Crit Care Med. 2008;36:1925–32.
Vary TC, Siegel JH, Nakatani T, Sato T, Aoyama H. Effect of sepsis on activity of pyruvate dehydrogenase complex in skeletal muscle and liver. Am J Physiol. 1986;250:E634–40.
Malaisse WJ, Nadi AB, Ladriere L, Zhang TM. Protective effects of succinic acid dimethyl ester infusion in experimental endotoxemia. Nutrition. 1997;13:330–41.
Gnaegi A, Feihl F, Boulat O, Waeber B, Liaudet L. Moderate hypercapnia exerts beneficial effects on splanchnic energy metabolism during endotoxemia. Intensive Care Med. 2009;35:1297–304.
Chvojka J, Sykora R, Krouzecky A, et al. Renal haemodynamic, microcirculatory, metabolic and histopathological responses to peritonitis-induced septic shock in pigs. Crit Care. 2008;12:R164.
Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640–50.
Astiz M, Rackow EC, Weil MH, Schumer W. Early impairment of oxidative metabolism and energy production in severe sepsis. Circ Shock. 1988;26:311–20.
Blennow G, Folbergrová J, Nilsson B, Siesjö BK. Cerebral metabolic and circulatory changes in the rat during sustained seizures induced by DL-homocysteine. Brain Res. 1979;179:129–46.
Folbergrová J, Jesina P, Drahota Z, et al. Mitochondrial complex I inhibition in cerebral cortex of immature rats following homocysteic acid-induced seizures. Exp Neurol. 2007;204:597–609.
Howse DCN. Metabolic responses to status epilepticus in the rat, cat, and mouse. Can J Physiol Pharmacol. 1979;57:205–12.
Folbergrová J, Ingvar M, Nevander G, Siesjö BK. Cerebral metabolic changes during and following fluorothyl-induced seizures in ventilated rats. J Neurochem. 1985;44:1419–26.
Ingvar M, Folbergrová J, Siesjö BK. Metabolic alterations underlying the development of hypermetabolic necrosis in the substantia nigra in status epilepticus. J Cereb Blood Flow Metab. 1987;7:103–8.
Chapman AG, Meldrum BS, Siesjö BK. Cerebral metabolic changes during prolonged epileptic seizures in rats. J Neurochem. 1977;28:1025–35.
Johansson BB, Fredriksson K. Cerebral energy metabolism during bicuculline-induced status epilepticus in spontaneously hypertensive rats. Acta Phys Scand. 1985;123:299–302.
Slais K, Vorisek I, Zoremba N, Homola A, Dmytrenko L, Sykova E. Brain metabolism and diffusion in the rat cerebral cortex during pilocarpine-induced status epilepticus. Exp Neurol. 2008;209:145–54.
Darbin O, Risso JJ, Carre E, Lonjon M, Naritoku DK. Metabolic changes in rat striatum following convulsive seizures. Brain Res. 2005;1050:124–9.
Claassen J, Jetté N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1256–65.
Folbergrová J, MacMillan V, Siesjö BK. The effect of moderate and marked hypercapnia upon the energy state and upon the cytoplasmic NADH-NAD+ ratio of the rat brain. J Neurochem. 1972;19:2497–505.
Folbergrová J, Pontén U, Siesjö BK. Patterns of changes in brain carbohydrate metabolites, amino acids and organic phosphates at increased carbon dioxide tensions. J Neurochem. 1974;22:1115–25.
Weyne J, Demeester G, Leusen I. Effects of carbon dioxide, bicarbonate, and pH on lactate and pyruvate in the brain of rats. Pflugers Arch. 1970;31:292–311.
Kjällquist Å, Nardini M, Siesjö BK. The regulation of extra- and intracellular acid-base parameters in the rat brain during hyper- and hypocapnia. Acta Physiol Scand. 1969;76:485–94.
Granholm L, Siesjö BK. The effects of hypercapnia and hypocapnia upon the cerebrospinal fluid lactate and pyruvate concentrations and upon the lactate, pyruvate, ATP, ADP, phosphocreatine and creatine concentrations of cat brain tissue. Acta Physiol Scand. 1969;75:257–66.
Frauendorf E, Hartmann N, Hübner G, Meng W, Weber A. Das verhalten des laktat/pyruvat-quotienten im rahmen moderner schilddrüsendiagnostik. Z Gesamte Inn Med. 1980;35:155–61.
Hübner G, Schwinger E, Meng W. Zum verhalten von laktat und pyruvat sowie des laktat/pyruvat-quotienten im blut bei schilddrüsenfunktionsstörungen des menschen. Z Gesamte Inn Med. 1975;30:786–9.
Katyare SS, Joshi MV, Fatterpaker P, Sreenivasan A. Effect of thyroid deficiency on oxidative phosphorylation in rat liver, kidney, and brain mitochondria. Arch Biochem Biophys. 1977;182:155–63.
Lecky FE, Little RA, Maycock PF, et al. Effect of alcohol on the lactate/pyruvate ratio of recently injured adults. Crit Care Med. 2002;30:981–5.
Yap M, Mascord DJ, Starmer GA, Whitfield JB. Studies on the chronopharmacology of ethanol. Alcohol Alcohol. 1993;28:17–24.
Myrsten AL, Rydberg U, Ideström CM, Lamble R. Alcohol intoxication and hangover: modification of hangover by chlormethiazole. Psychopharmacology (Berl). 1980;69:117–25.
Ginestal da Cruz A, Correia JP, Menezes L. Ethanol metabolism in liver cirrhosis and chronic alcoholism. Acta Hepatogastroenterol (Stuttg). 1975;22:369–74.
Frayn KN, Coppack SW, Walsh PE, Butterworth HC, Humphreys SM, Pedrosa HC. Metabolic responses of forearm and adipose tissues to acute ethanol ingestion. Metabolism. 1990;39:958–66.
Hollstedt C, Rydberg U, Olsson O, Buijten J. Effects of ethanol on the developing rat. I. Ethanol metabolism and effects on lactate, pyruvate, and glucose concentrations. Med Biol. 1980;58:158–63.
Pronko PS, Velichko MG, Moroz AR, Rubanovich NN. Low-molecular-weight metabolites relevant to ethanol metabolism: correlation with alcohol withdrawal severity and utility for identification of alcoholics. Alcohol Alcohol. 1997;32:761–8.
Krebs HA, Freedland RA, Hems R, Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969;112:117–24.
Tskuamoto S, Kanegae T, Saito M, et al. Concentrations of blood and urine ethanol, acetaldehyde, acetate and acetone during experimental hangover in volunteers. Arukoru Kenkyuto Yakubutsu Ison. 1991;26:500–10.
Martin E, Rosenthal RE, Fiskum G. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res. 2005;79:240–7.
Ehrig K, Heckel R, Lajios G. Molecular analysis of metabolic pathway with graph transformation. Lect Notes Comput Sci. 2006;4178:107–21.
Acknowledgments
The authors gratefully acknowledge the helpful and insightful suggestions of Julien F. Biebuyck, David F. Wilson, Gretchen L. Redline, Marilyn G. Larach, and Sarah M. Gibbs in the preparation of the manuscript. DBL received financial support from the Clinical Neurosciences Training Program (CNST) Summer Research Fellowship program of the University of Pennsylvania School of Medicine.
Disclosure
PLR has received support and research funding from Integra LifeSciences (Plainsboro, NJ) and CMA Microdialysis (Solna, Sweden). WAK has received support and research funding from Covidien (Mansfield, MA) and the U.S. Public Health Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larach, D.B., Kofke, W.A. & Le Roux, P. Potential Non-Hypoxic/Ischemic Causes of Increased Cerebral Interstitial Fluid Lactate/Pyruvate Ratio: A Review of Available Literature. Neurocrit Care 15, 609–622 (2011). https://doi.org/10.1007/s12028-011-9517-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9517-8