Abstract
Purpose
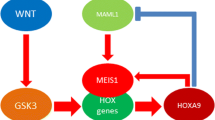
Homeobox (HOX) transcription factors are critical regulators of cell fate, stem cell functions, and gastrointestinal development. They require three-amino acid loop extension (TALE) homeodomain proteins such as Meis1 to enhance their transcriptional efficiencies. There are complicated associations between different signaling pathways such as the Wnt and NOTCH and tumor progression. It has been investigated that GSK-3 as an important component of the Wnt pathway facilitates the expression of HOX target genes. Therefore, in the present study, we assessed the probable correlation between Wnt, NOTCH, and HOX genes in esophageal squamous cell carcinoma (ESCC) progression and metastasis through the correlational study between the Msi1 as an important activator for both of the NOTCH and Wnt pathways and Meis1.
Methods
Levels of Meis1 and Msi1 messenger RNA (mRNA) expression in 51 ESCC patients were compared to the normal tissues using real-time polymerase chain reaction.
Results
Only 3 out of 51 (5.9 %) cases had Meis1/Msi1 overexpression and also 3/51 (5.9 %) cases had Meis1/Msi1 underexpression. There was a significant correlation between the Msi1 and Mesi1 mRNA expression (p = 0.037). All of the Msi1/Meis1 underexpressed tumors were poorly differentiated (p = 0.003). Meis1 under/Msi1 overexpressed cases also were in T3 tumor depth of invasion (p = 0.019). And there was a significant correlation between the Msi1/Meis1 underexpression and gender (p = 0.045).
Conclusions
Our results show that Meis1 may have a positive feedback with Msi1 during the ESCC progression.

Similar content being viewed by others
References
Owens BM, Hawley RG. HOX and non-HOX homeobox genes in leukemic hematopoiesis. Stem Cells. 2002;20(5):364–79.
Sitwala KV, Dandekar MN, Hess JL. HOX proteins and leukemia. Int J Clin Exp Pathol. 2008;1(6):461–74.
Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25(21):4173–80.
Fognani C, Kilstrup-Nielsen C, Berthelsen J, Ferretti E, Zappavigna V, Blasi F. Characterization of PREP2, a paralog of PREP1, which defines a novel sub-family of the MEINOX TALE homeodomain transcription factors. Nucleic Acids Res. 2002;30(9):2043–51.
Monica K, Galili N, Nourse J, Saltman D, Cleary ML. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol. 1991;11(12):6149–57.
Wang K, Yin XM, Copeland NG, Gilbert DJ, Jenkins NA, Keck CL, et al. BID, a proapoptotic BCL-2 family member, is localized to mouse chromosome 6 and human chromosome 22q11. Genomics. 1998;53(2):235–8.
DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, et al. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98(3):618–26.
Pillay LM, Forrester AM, Erickson T, Berman JN, Waskiewicz AJ. The Hox cofactors Meis1 and Pbx act upstream of gata1 to regulate primitive hematopoiesis. Dev Biol. 2010;340(2):306–17.
Goh SL, Looi Y, Shen H, Fang J, Bodner C, Houle M, et al. Transcriptional activation by MEIS1A in response to protein kinase A signaling requires the transducers of regulated CREB family of CREB co-activators. J Biol Chem. 2009;284(28):18904–12.
Huang H, Rastegar M, Bodner C, Goh SL, Rambaldi I, Featherstone M. MEIS C termini harbor transcriptional activation domains that respond to cell signaling. J Biol Chem. 2005;280(11):10119–27.
Li J, Shen H, Himmel KL, Dupuy AJ, Largaespada DA, Nakamura T, et al. Leukaemia disease genes: large-scale cloning and pathway predictions. Nat Genet. 1999;23(3):348–53.
Nakamura T, Largaespada DA, Shaughnessy Jr JD, Jenkins NA, Copeland NG. Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukaemias. Nat Genet. 1996;12(2):149–53.
Esparza SD, Chang J, Shankar DB, Zhang B, Nelson SF, Sakamoto KM. CREB regulates Meis1 expression in normal and malignant hematopoietic cells. Leukemia. 2008;22(3):665–7.
Ferretti E, Villaescusa JC, Di Rosa P, Fernandez-Diaz LC, Longobardi E, Mazzieri R, et al. Hypomorphic mutation of the TALE gene Prep1 (pKnox1) causes a major reduction of Pbx and Meis proteins and a pleiotropic embryonic phenotype. Mol Cell Biol. 2006;26(15):5650–62.
Hu YL, Fong S, Ferrell C, Largman C, Shen WF. HOXA9 modulates its oncogenic partner Meis1 to influence normal hematopoiesis. Mol Cell Biol. 2009;29(18):5181–92.
Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH, et al. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell. 2010;17(6):597–608.
Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21WAF-1. Mol Cell Neurosci. 2006;31(1):85–96.
Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21(12):3888–900.
Moghbeli M, Forghanifard MM, Aarabi A, Mansourian A, Abbaszadegan MR. Clinicopathological sex-related relevance of musashi1 mRNA expression in esophageal squamous cell carcinoma patients. Pathol Oncol Res. 2014;20(2):427–33.
Rad A, Farshchian M, Forghanifard MM, Matin MM, Bahrami AR, Geerts D et al. Predicting the molecular role of MEIS1 in esophageal squamous cell carcinoma. Tumour Biol. 2015.
Chen H, Chung S, Sukumar S. HOXA5-induced apoptosis in breast cancer cells is mediated by caspases 2 and 8. Mol Cell Biol. 2004;24(2):924–35.
Grier DG, Thompson A, Kwasniewska A, McGonigle GJ, Halliday HL, Lappin TR. The pathophysiology of HOX genes and their role in cancer. J Pathol. 2005;205(2):154–71.
Ji Q, Liu PI, Chen PK, Aoyama C. Follicle stimulating hormone-induced growth promotion and gene expression profiles on ovarian surface epithelial cells. Int J Cancer. 2004;112(5):803–14.
Riz I, Hawley RG. G1/S transcriptional networks modulated by the HOX11/TLX1 oncogene of T-cell acute lymphoblastic leukemia. Oncogene. 2005;24(36):5561–75.
Yamashita T, Tazawa S, Yawei Z, Katayama H, Kato Y, Nishiwaki K, et al. Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol. 2006;28(4):931–8.
Calvo KR, Knoepfler PS, Sykes DB, Pasillas MP, Kamps MP. Meis1a suppresses differentiation by G-CSF and promotes proliferation by SCF: potential mechanisms of cooperativity with Hoxa9 in myeloid leukemia. Proc Natl Acad Sci U S A. 2001;98(23):13120–5.
Argiropoulos B, Yung E, Humphries RK. Unraveling the crucial roles of Meis1 in leukemogenesis and normal hematopoiesis. Genes Dev. 2007;21(22):2845–9.
Kumar AR, Li Q, Hudson WA, Chen W, Sam T, Yao Q, et al. A role for MEIS1 in MLL-fusion gene leukemia. Blood. 2009;113(8):1756–8.
Acknowledgments
This work was supported by a grant from the Vice Chancellor for Research at Mashhad University of Medical Sciences and was part of a Ph.D. student’s dissertation, No. 921202 and 89751.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Moghbeli, M., Rad, A., Farshchian, M. et al. Correlation Between Meis1 and Msi1 in Esophageal Squamous Cell Carcinoma. J Gastrointest Canc 47, 273–277 (2016). https://doi.org/10.1007/s12029-016-9824-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-016-9824-6