Abstract
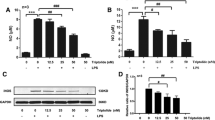
To further understand the anti-inflammatory effect of adenosine cyclic 3′,5′-monophosphate (cAMP), we examined the effect of protein kinase A (PKA) and cAMP-responsive guanine nucleotide exchange factor (Epac) on the transcription and production of cytokines and on the activity of mitogen-activated protein kinases (MAPK) p38 and glycogen synthase kinase-3β (GSK-3β) in endotoxin-treated rat primary cultured microglia. The PKA specific agonist N6-benzoyladenosine-3,5-cAMP (6-Bnz-cAMP) not only inhibited the transcription and production of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) but also enhanced the transcription and expression of IL-10, while the Epac selective analog 8-(4-chlorophenylthio)-2-O-methyladenosine-3,5-cAMP (8-pCPT-2′-O-Me-cAMP) merely repressed the TNF-α expression. Western blots assays indicated that 6-Bnz-cAMP significantly inhibited lipopolysaccharide-induced activation of both p38 and GSK-3β in a dose-dependent manner; in contrast, 8-pCPT-2′-O-Me-cAMP only slightly repressed GSK-3β activity at large doses. Pretreatment with H-89, a specific PKA antagonist, could completely reverse the effect of 6-Bnz-cAMP on cytokines expressions and kinases activities but had no effect on the performance of 8-pCPT-2′-O-Me-cAMP. Our findings indicate that PKA and Epac exert differential effect on the expression of inflammatory cytokines such as TNF-α, IL-1β, and IL-10, possibly owing to the different effects on the downstream effectors, MAPK p38, and GSK-3β.




Similar content being viewed by others
References
Aloisi F (2001) Immune function of microglia. Glia 36:165–179
Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M (2005) Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174:595–599
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440
Bhat NR, Zhang P, Lee JC, Hogan EL (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18:1633–1641
Bos JL (2003) Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4:733–738
Cheng YL, Wang CY, Huang WC et al (2009) Staphylococcus aureus induces microglial inflammation via a glycogen synthase kinase 3beta-regulated pathway. Infect Immun 77:4002–4008
Dugo L, Collin M, Thiemermann C (2007) Glycogen synthase kinase 3beta as a target for the therapy of shock and inflammation. Shock 27:113–123
Enserink JM, Christensen AE, de Rooij J et al (2001) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906
Garden GA, Möller T (2006) Microglia biology in health and disease. J Neuroimmune Pharmacol 1:127–137
Huang WC, LinYS WCY et al (2009) Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 128:e275–e286
Jing H, Yen JH, Ganea D (2004) A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem 279:55176–55186
Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–869
Lee H, Suk K (2004) Selective modulation of microglial signal transduction by PACAP. NeuroReport 15:1469–1474
Lucin KM, Wyss-Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64:110–122
Miller G (2005) Neuroscience. The dark side of glia. Science 308:778–781
Moon EY, Oh SY, Han GH, Lee CS, Park SK (2005) Epac1-mediated Rap1 activation is not required for the production of nitric oxide in BV2, murine microglial cells. J Neurosci Res 81:38–44
Nakamura Y, Si QS, Kataoka K (1999) Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35:95–100
Petrova TV, Akama KT, Van Eldik LJ (1999) Selective modulation of BV2 microglial activation by prostaglandin E2. Differential effects on endotoxin-stimulated cytokine induction. J Biol Chem 274:28823–28827
Woo MS, Jang PG, Park JS, Kim WK, Joh TH, Kim HS (2003) Selective modulation of lipopolysaccharide-stimulated cytokine expression and mitogen-activated protein kinase pathways by dibutytyl-cAMP in BV2 microglial cells. Mol Brain Res 113:86–96
Woo MS, Jung SH, Hyun JW, Kim HS (2004) Differential regulation of inducible nitric oxide synthase and cytokine gene expression by forskolin and dibutyryl-cAMP in lipopolysaccharide-stimulated murine BV2 microglial cells. Neurosci Lett 356:187–190
Xue Y, Wang Y, Feng DC, Xiao BG, Xu LY (2008) Tetrandrine suppresses lipopolysaccharide induced microglial activation by inhibiting NF-kappaB pathway. Acta Pharmacol Sin 29:245–251
Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M (1999) Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler 5:126–133
Zhang B, Yang L, Konishi Y, Maeda N, Sakanaka M, Tanaka J (2002) Suppressive effects of phosphodiesterase type IV inhibitors in rat cultured microglial cells: comparison with other types of cAMP elevating agents. Neuropharmacology 42:262–269
Zhang J, Bui TN, Xiang J, Lin A (2006) Cyclic AMP inhibits p38 activation via CREB-induced dynein light chain. Mol Cell Biol 26:1223–1234
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30600583 and 30901399) and National Postdoctoral Science Foundation of China (20080440625). We thank Dr. Yanxia Wang and Yong Li for assistance in microglia culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Zhao, X., Cao, J. et al. Differential Roles of PKA and Epac on the Production of Cytokines in the Endotoxin-Stimulated Primary Cultured Microglia. J Mol Neurosci 45, 186–193 (2011). https://doi.org/10.1007/s12031-010-9426-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9426-x