Abstract
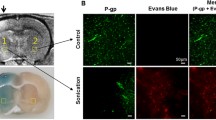
This research was designed to determine whether disrupting the blood–brain barrier (BBB) in rats by applying focused ultrasound (FUS) combined with microbubbles induced changes in the density of caveolae and/or the expression of the structural protein caveolin-1. To this end, two approaches were utilized. First, using enhanced magnetic resonance imaging, characteristics of BBB disruption induced by our specific FUS parameters and dose of microbubble were recorded, and the time after treatment when the BBB was the most permeable was determined. Second, rats were treated with FUS or microbubbles alone, both or neither, and a combination of Evans blue (EB) BBB permeability assays, streptavidin–peroxidase (SP) immunohistochemistry, western blot, and transmission electron microscopy (TEM) was employed to detect any changes in caveolae density and caveolin-1 expression at the previously determined time point when the BBB was the most permeable. The first set of studies revealed that our specific FUS parameters and dose of microbubbles were able to induce a transient, targeted, and reversible BBB opening in rats, and that the BBB was the most permeable 1 h after treatment with FUS and microbubbles. In the second set of experiments, the results of the SP immunohistochemistry, western blot, and TEM, taken together, revealed that caveolae and caveolin-1 were primarily localized in the brain microvascular endothelial cells of all of the rats regardless of treatment, and that caveolin-1 expression was highest in the rats treated with both FUS and microbubbles. In summary, treatment with FUS, in combination with a dose of microbubbles, can enhance BBB permeability through a caveolae-mediated transcellular approach by upregulating the expression level of caveolin-1 and, consequently, the amount of caveolae. This caveolin-1-mediated transcellular transport pathway may cooperate with other transport pathways to induce opening of the BBB. This research sheds light on the mechanism of a transient, targeted, and reversible opening of the BBB induced by FUS combined with microbubbles.







Similar content being viewed by others
References
Anderson RG (1998) The caveolae membrane system. Annu Rev Biochem 67:199–225
Balda MS, Flores-Maldonado C, Cereijido M, Matter K (2000) Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem 78(1):85–96
Coomber BL, Stewart PA (1985) Morphometric analysis of CNS microvascular endothelium. Microvasc Res 30(1):99–115
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560
Deng CX, Sieling F, Pan H, Cui J (2004) Ultrasound-induced cell membrane porosity. Ultrasound Med Biol 30(4):519–526
Doolittle ND, Miner ME, Hall WA et al (2000) Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood–brain barrier for the treatment of patients with malignant brain tumors. Cancer 88(3):637–647
Frank PG, Woodman SE, Park DS, Lisanti MP (2003) Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 23(7):1161–1168
Gonzalez-Mariscal L, Betanzos A, Avila-Flores A (2000) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11(4):315–324
Gu YT, Xue YX, Zhang H, Li Y, Liang XY (2011) Adenosine 5′-triphosphate-sensitive potassium channel activator induces the up-regulation of caveolin-1 expression in a rat brain tumor model. Cell Mol Neurobiol 31(4):629–634
Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H (2004) Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest New Drugs 22(1):27–37
Hilgenfeldt S, Lohse D, Zomack M (2000) Sound scattering and localized heat deposition of pulse-driven microbubbles. J Acoust Soc Am 107(6):3530–3539
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging-guided focal opening of the blood–brain barrier in rabbits. Radiology 220(3):640–646
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2003) Non-invasive opening of BBB by focused ultrasound. Acta Neurochir 86(Suppl):555–558
Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N (2005) Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 24(1):12–20
Hynynen K, McDannold N, Vykhodtseva N et al (2006) Focal disruption of the blood–brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg 105(3):445–454
Ikezu T, Ueda H, Trapp BD et al (1998) Affinity-purification and characterization of caveolins from the brain: differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res 804(2):177–192
Janigro D, West GA, Gordon EL, Winn HR (1993) ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am J Physiol 265(3 Pt 1):C812–C821
Kinoshita M (2006) Targeted drug delivery to the brain using focused ultrasound. Top Magn Reson Imaging 17(3):209–215
Kinoshita M, McDannold N, Jolesz FA, Hynynen K (2006) Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 340(4):1085–1090
Kroll RA, Neuwelt EA (1998) Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery 42(5):1083–1099 (discussion 1099–1100)
Lamaze C, Schmid SL (1995) The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol 7(4):573–580
Lionetti V, Fittipaldi A, Agostini S, Giacca M, Recchia FA, Picano E (2009) Enhanced caveolae-mediated endocytosis by diagnostic ultrasound in vitro. Ultrasound Med Biol 35(1):136–143
McDannold N, Vykhodtseva N, Hynynen K (2006) Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol 51(4):793–807
Mei J, Cheng Y, Song Y et al (2009) Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med 28(7):871–880
Meijering BD, Juffermans LJ, van Wamel A et al (2009) Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res 104(5):679–687
Nag S (2003) Pathophysiology of blood–brain barrier breakdown. Methods Mol Med 89:97–119
Nag S, Venugopalan R, Stewart DJ (2007) Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood–brain barrier breakdown. Acta Neuropathol 114(5):459–469
Niwa K, Sakai J, Karino T et al (2006) Reactive oxygen species mediate shear stress-induced fluid-phase endocytosis in vascular endothelial cells. Free Radic Res 40(2):167–174
Palade GE (1953) Fine structure of blood capillaries. J Appl Phys 24:1424–1436
Pardridge WM (2002a) Drug and gene delivery to the brain: the vascular route. Neuron 36(4):555–558
Pardridge WM (2002b) Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov 1(2):131–139
Pardridge WM (2003) Blood–brain barrier genomics and the use of endogenous transporters to cause drug penetration into the brain. Curr Opin Drug Discov Dev 6(5):683–691
Park J, Fan Z, Kumon RE, El-Sayed ME, Deng CX (2010) Modulation of intracellular Ca2+ concentration in brain microvascular endothelial cells in vitro by acoustic cavitation. Ultrasound Med Biol 36(7):1176–1187
Rubin LL, Staddon JM (1999) The cell biology of the blood–brain barrier. Ann Rev Neurosci 22:11–28
Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K (2004) Cellular mechanisms of the blood–brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 30(7):979–989
Sheikov N, McDannold N, Sharma S, Hynynen K (2008) Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol 34(7):1093–1104
Stan RV (2005) Structure of caveolae. Biochim Biophys Acta 1746(3):334–348
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2(4):285–293
Virgintino D, Robertson D, Errede M et al (2002) Expression of caveolin-1 in human brain microvessels. Neuroscience 115(1):145–152
Wang F, Cheng Y, Mei J et al (2009) Focused ultrasound microbubble destruction-mediated changes in blood–brain barrier permeability assessed by contrast-enhanced magnetic resonance imaging. J Ultrasound Med 28(11):1501–1509
Xia CY, Zhang Z, Xue YX, Wang P, Liu YH (2009) Mechanisms of the increase in the permeability of the blood–tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J Neurooncol 94(1):41–50
Yu J, Bergaya S, Murata T et al (2006) Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116(5):1284–1291
Acknowledgments
The authors thank the Chongqing Haifu (HIFU) Technology Co. Ltd. for providing essential help. This work is supported by National Natural Science Foundation of China (No. 30870723).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, J., Huang, Q., Wang, F. et al. The Role of Caveolin-1 in Blood–Brain Barrier Disruption Induced by Focused Ultrasound Combined with Microbubbles. J Mol Neurosci 46, 677–687 (2012). https://doi.org/10.1007/s12031-011-9629-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9629-9