Abstract
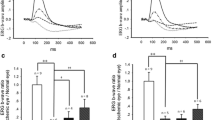
A number of studies have proven that pituitary adenylate cyclase activating polypeptide (PACAP) is protective in neurodegenerative diseases. Permanent bilateral common carotid artery occlusion (BCCAO) causes severe degeneration in the rat retina. In our previous studies, protective effects were observed with PACAP1-38, PACAP1-27, and VIP but not with their related peptides, glucagon, or secretin in BCCAO. All three PACAP receptors (PAC1, VPAC1, VPAC2) appear in the retina. Molecular and immunohistochemical analysis demonstrated that the retinoprotective effects are most probably mainly mediated by the PAC1 receptor. The aim of the present study was to investigate the retinoprotective effects of a selective PAC1-receptor agonist maxadilan in BCCAO-induced retinopathy. Wistar rats were used in the experiment. After performing BCCAO, the right eye was treated with intravitreal maxadilan (0.1 or 1 μM), while the left eye was injected with vehicle. Sham-operated rats received the same treatment. Two weeks after the operation, retinas were processed for standard morphometric and molecular analysis. Intravitreal injection of 0.1 or 1 μM maxadilan caused significant protection in the thickness of most retinal layers and the number of cells in the GCL compared to the BCCAO-operated eyes. In addition, 1 μM maxadilan application was more effective than 0.1 μM maxadilan treatment in the ONL, INL, IPL, and the entire retina (OLM-ILM). Maxadilan treatment significantly decreased cytokine expression (CINC-1, IL-1α, and L-selectin) in ischemia. In summary, our histological and molecular analysis showed that maxadilan, a selective PAC1 receptor agonist, has a protective role in BCCAO-induced retinal degeneration, further supporting the role of PAC1 receptor conveying the retinoprotective effects of PACAP.




Similar content being viewed by others
References
Atlasz T, Babai N, Kiss P et al (2007a) Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen Comp Endocrinol 153:108–114
Atlasz T, Babai N, Reglodi D et al (2007b) Diazoxide is protective in the rat retina against ischemic injury induced by bilateral carotid occlusion and glutamate-induced degeneration. Neurotox Res 12:105–111
Atlasz T, Szabadfi K, Kiss P et al (2010) Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res Bull 81:497–504
Atlasz T, Szabadfi K, Kiss P et al (2011) Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J Mol Neurosci 43:51–57
Atlasz T, Vaczy A, Werling D et al (2016) In: Reglodi D, Tamas A (eds) Neuroprotective effects of PACAP in the retina. Springer Nature, New York, pp 501–527
Babai N, Atlasz T, Tamas A et al (2006) Search for the optimal monosodium glutamate treatment schedule to study the neuroprotective effects of PACAP in the retina. Ann N Y Acad Sci 1070:149–155
Banki E, Degrell P, Kiss P et al (2013) Effect of PACAP treatment on kidney morphology and cytokine expression in rat diabetic nephropathy. Peptides 42:125–130
Banki E, Hajna Z, Kemeny A et al (2014) The selective PAC1 receptor agonist maxadilan inhibits neurogenic vasodilation and edema formation in the mouse skin. Neuropharmacology 85:538–547
Bourgault S, Vaudry D, Botia B et al (2008) Novel stable PACAP analogs with potent activity towards the PAC1 receptor. Peptides 29:919–932
Chapter MC, White CM, DeRidder A et al (2010) Chemical modification of class II G protein-coupled receptor ligands: Frontiers in the development of peptide analogs as neuroendocrine pharmacological therapies. Pharmacol Ther 125:39–54
Clason TA, Girard BM, May V, Parsons RL (2016) Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. J Mol Neurosci 59:309–316
Cohen I, Rider P, Carmi Y et al (2010) Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A 107:2574–2579
Couvineau A, Laburthe M (2012) VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol 166:42–50
D’Amico AG, Maugeri G, Reitano R et al (2015) PACAP modulates expression of hypoxia-inducible factors in streptozotocin-induced diabetic rat retina. J Mol Neurosci 57:501–509
Danyadi B, Szabadfi K, Reglodi D et al (2014) PACAP application improves functional outcome of chronic retinal ischemic injury in rats-evidence from electroretinographic measurements. J Mol Neurosci 54:293–299
Dejda A, Seaborn T, Bourgault S et al (2011) PACAP and a novel stable analog protect rat brain from ischemia: insight into the mechanisms of action. Peptides 32:1207–1216
Dinarello CA (1994) The interleukin-1 family: 10 years of discovery. FASEB J 8:1314–1325
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732
Dreixler JC, Hemmert JW, Shenoy SK et al (2009) The role of Akt/protein kinase B subtypes in retinal ischemic preconditioning. Exp Eye Res 88:512–521
Endo K, Nakamachi T, Seki T et al (2011) Neuroprotective effect of PACAP against NMDA-induced retinal damage in the mouse. J Mol Neurosci 43:22–29
Farkas E, Luiten PGMM, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
Ghaly A, Marsh DR (2010) Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int J Exp Pathol 91:244–255
Grevelink SA, Osborne J, Loscalzo J, Lerner EA (1995) Vasorelaxant and second messenger effects of maxadilan. J Pharmacol Exp Ther 272:33–37
Guilpin VO, Swardson-Olver C, Nosbisch L, Titus RG (2002) Maxadilan, the vasodilator/immunomodulator from Lutzomyia longipalpis sand fly saliva, stimulates haematopoiesis in mice. Parasite Immunol 24:437–446
Guo X, Yu R, Xu Y et al (2016) PAC1R agonist maxadilan enhances hADSC viability and neural differentiation potential. J Cell Mol Med 10:1–17
Harmar AJ, Sheward WJ, Morrison CF et al (2004) Distribution of the VPAC 2 receptor in peripheral tissues of the mouse. Endocrinology 145:1203–1210
Hashimoto H (2002) Physiological significance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the nervous system. Yakugaku Zasshi 122:1109–1121
Hoover DB, Tompkins JD, Parsons RL (2009) Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27). J Pharmacol Exp Ther 331:197–203
Horvath G, Racz B, Reglodi D et al (2010) Effects of PACAP on mitochondrial apoptotic pathways and cytokine expression in rats subjected to renal ischemia/reperfusion. J Mol Neurosci 42:411–418
Jackson TS, Lerner E, Weisbrod RM et al (1996) Vasodilatory properties of recombinant maxadilan. Am J Phys 271:H924–H930
Jander S, Sitzer M, Schumann R et al (1998) Inflammation in high-grade carotid stenosis: a possible role for macrophages and T cells in plaque destabilization. Stroke 29:1625–1630
Juhasz T, Matta C, Katona E et al (2014) Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line. J Mol Neurosci 54:555–573
Juhasz T, Helgadottir SL, Tamas A et al (2015) PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 66:51–57
Junnarkar SP, Tapuria N, Mani A et al (2010) Attenuation of warm ischemia-reperfusion injury in the liver by bucillamine through decreased neutrophil activation and Bax/Bcl-2 modulation. J Gastroenterol Hepatol 25:1891–1899
Kambe Y, Miyata A (2012) Role of mitochondrial activation in PACAP dependent neurite outgrowth. J Mol Neurosci 48:550–557
Kanekar S, Gandham M, Lucero MT (2010) PACAP protects against TNFα-induced cell death in olfactory epithelium and olfactory placodal cell lines. Mol Cell Neurosci 45:345–354
Krajcs N, Hernadi L, Pirger Z et al (2015) PACAP modulates acetylcholine-elicited contractions at nicotinic neuromuscular contacts of the land snail. J Mol Neurosci 57:492–500
Kubrusly RCC, da Cunha MCC, de Reis RAM et al (2005) Expression of functional receptors and transmitter enzymes in cultured Muller cells. Brain Res 1038:141–149
Kvarik T, Mammel B, Reglodi D et al (2016) PACAP is protective in a rat model of retinopathy of prematurity. J Mol Neurosci (in press)
Lamacchia C, Rodriguez E, Palmer G, Gabay C (2013) Endogenous IL-1α is a chromatin-associated protein in mouse macrophages. Cytokine 63:135–144
Laszlo E, Varga A, Kovacs K et al (2015) Ischemia/reperfusion-induced kidney injury in heterozygous PACAP-deficient mice. Transplant Proc 47:2210–2215
Lerner EA, Iuga AO, Reddy VB et al (2007) Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 28:1651–1654
Matsumoto M, Nakamachi T, Watanabe J et al (2016) Pituitary adenylate cyclase-activating polypeptide (PACAP) is involved in adult mouse hippocampal neurogenesis after stroke. J Mol Neurosci 59:270–279
Mester L, Szabo A, Atlasz T et al (2009) Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI-3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox Res 16:68–76
Miyata A, Arimura A, Dahl RR et al (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164:567–574
Moro O, Lerner EA (1997) Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem 272:966–970
Moro O, Tajima M, Lerner EA (1996) Receptors for the vasodilator maxadilan are expressed on selected neural crest and smooth muscle-derived cells. Insect Biochem Mol Biol 26:1019–1025
Moro O, Wakita K, Ohnuma M et al (1999) Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem 274:23103–23110
Morris RV, Shoemaker CB, David JR et al (2001) Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol 167:5226–5230
Nedvig K, Weber G, Nemeth J et al (2012) Changes of PACAP immunoreactivities and cytokine levels after PACAP-38 containing intestinal preservation and autotransplantation. J Mol Neurosci 48:788–794
Njaine B, Martins RAP, Santiago MF et al (2010) Pituitary adenylyl cyclase-activating polypeptide controls the proliferation of retinal progenitor cells through downregulation of cyclin D1. Eur J Neurosci 32:311–321
Ohnou T, Yokai M, Kurihara T et al (2016) Pituitary adenylate cyclase-activating polypeptide type 1 receptor signaling evokes long-lasting nociceptive behaviors through the activation of spinal astrocytes in mice. J Pharmacol Sci 130:194–203
Padua D, Vu JP, Germano PM, Pisegna JR (2016) The role of neuropeptides in mouse models of colitis. J Mol Neurosci 59:203–210
Ramos-Álvarez I, Mantey SA, Nakamura T et al (2015) A structure–function study of PACAP using conformationally restricted analogs: identification of PAC1 receptor-selective PACAP agonists. Peptides 66:26–42
Reglodi D, Borzsei R, Bagoly T et al (2008) Agonistic behavior of PACAP6-38 on sensory nerve terminals and cytotrophoblast cells. J Mol Neurosci 36:270–278
Reglodi D, Kiss P, Szabadfi K et al (2012) PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J Mol Neurosci 48:482–492
Reglodi D, Renaud J, Tamas A et al (2015) Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol S0301-0082(15):00128–00128. doi:10.1016/j.pneurobio.2015.10.004
Robberecht P, Gourlet P, De Neef P et al (1992) Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6-38) as a potent antagonist. Eur J Biochem 207:239–246
Scuderi S, D’Amico AG, Castorina A et al (2013) Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides 39:119–124
Scuderi S, D’amico AG, Federico C et al (2015) Different retinal expression patterns of IL-1α, IL-1β, and their receptors in a rat model of type 1 STZ-induced diabetes. J Mol Neurosci 56:431–439
Seki T, Shioda S, Ogino D et al (1997) Distribution and ultrastructural localization of a receptor for pituitary adenylate cyclase activating polypeptide and its mRNA in the rat retina. Neurosci Lett 238:127–130
Seki T, Izumi S, Shioda S et al (2000) Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Ann N Y Acad Sci 921:366–369
Seki T, Hinohara Y, Taki C et al (2006) PACAP stimulates the release of interleukin-6 in cultured rat Müller cells. Ann N Y Acad Sci 1070:535–539
Seki T, Itoh H, Nakamachi T, Shioda S (2008) Suppression of ganglion cell death by PACAP following optic nerve transection in the rat. J Mol Neurosci 36:57–60
Seki T, Itoh H, Nakamachi T et al (2011) Suppression of rat retinal ganglion cell death by PACAP following transient ischemia induced by high intraocular pressure. J Mol Neurosci 43:30–34
Shioda S, Nakamachi T (2015) PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 72:202–207
Shioda S, Takenoya F, Wada N et al (2016) Pleiotropic and retinoprotective functions of PACAP. Anat Sci Int 91:313–324
Shoge K, Mishima HK, Saitoh T et al (1998) Protective effects of vasoactive intestinal peptide against delayed glutamate neurotoxicity in cultured retina. Brain Res 809:127–136
Silveira MS, Costa MR, Bozza M, Linden R (2002) Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J Biol Chem 277:16075–16080
Soares MB, Titus RG, Shoemaker CB et al (1998) The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J Immunol 160:1811–1816
Sun L, Xu Y-W, Han J et al (2015) 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARγ: a possible neuroprotective effect in ischemic brain. J Lipid Res 56:502–514
Szabadfi K, Atlasz T, Reglodi D et al (2009) Urocortin 2 protects against retinal degeneration following bilateral common carotid artery occlusion in the rat. Neurosci Lett 455:42–45
Szabadfi K, Mester L, Reglodi D et al (2010) Novel neuroprotective strategies in ischemic retinal lesions. Int J Mol Sci 11:544–561
Szabadfi K, Atlasz T, Kiss P et al (2012a) Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox Res 21:41–48
Szabadfi K, Danyadi B, Kiss P et al (2012b) Preconditioning with volatile anaesthetic sevoflurane in ischemic retinal lesion in rats. J Mol Histol 43:565–569
Szabadfi K, Reglodi D, Szabo A et al (2016) Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: focus on the vertical information processing pathway. Neurotox Res 29:432–446
Szabo A, Danyadi B, Bognar E et al (2012) Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci Lett 523:93–98
Tamas A, Javorhazy A, Reglodi D et al (2016) Examination of PACAP-like immunoreactivity in urogenital tumor samples. J Mol Neurosci 59:177–183
Toker E, Kazokoğlu H, Sahin S (1998) Cell adhesion molecules in subretinal fluid: soluble forms of VCAM-1 (vascular cell adhesion molecule-1) and L-selectin. Int Ophthalmol 22:71–76
Tunçel N, Başmak H, Uzuner K et al (1996) Protection of rat retina from ischemia-reperfusion injury by vasoactive intestinal peptide (VIP): the effect of VIP on lipid peroxidation and antioxidant enzyme activity of retina and choroid. Ann N Y Acad Sci 805:489–498
Uchida D, Tatsuno I, Tanaka T et al (1998) Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP type 1 receptor. Ann N Y Acad Sci 865:253–258
Vollmar B, Menger MD (2011) Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbeck's Arch Surg 396:13–29
Vu JP, Larauche M, Flores M et al (2015) Regulation of appetite, body composition, and metabolic hormones by vasoactive intestinal polypeptide (VIP). J Mol Neurosci 56:377–387
Werling D, Reglodi D, Kiss P et al (2014) Investigation of PACAP fragments and related peptides in chronic retinal hypoperfusion. J Ophthalmol 2014:1–7
Wojcieszak J, Zawilska JB (2014) PACAP38 and PACAP6-38 exert cytotoxic activity against human retinoblastoma Y79 cells. J Mol Neurosci 54:463–468
Yoshida A, Yoshida S, Hata Y et al (1998) The role of NF-kappaB in retinal neovascularization in the rat. Possible involvement of cytokine-induced neutrophil chemoattractant (CINC), a member of the interleukin-8 family. J Histochem Cytochem 46:429–436
Acknowledgments
This study was supported by OTKA K104984, K119759, Bolyai Scholarship of the Hungarian Academy of Sciences, PTE AOK Research Grant, National Brain Research Program KTIA_13_NAP-A-III/5., “New National Excellence Program,” Astellas Foundation 2016, The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pecs, Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaczy, A., Reglodi, D., Somoskeoy, T. et al. The Protective Role of PAC1-Receptor Agonist Maxadilan in BCCAO-Induced Retinal Degeneration. J Mol Neurosci 60, 186–194 (2016). https://doi.org/10.1007/s12031-016-0818-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0818-4