Abstract
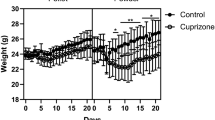
The chopper chelator cuprizone serves as a valuable chemical tool to induce consistent and reproducible demyelination in the central nervous system. However, the daily preparation of fresh cuprizone powder mixed in finely ground rodent chow might well be a particular health problem. Alternative methods, such as the fabrication of cuprizone-containing pellets, are available. The effectiveness of this method is, however, not known. In the present study, we compared whether intoxication of C57BL/6 mice with 0.25% cuprizone mixed into ground rodent chow does induce demyelination to a similar extent compared to a cuprizone-pellet intoxication protocol. We found that feeding of 0.25% cuprizone in ground chow provides a strong, well-defined, and reproducible demyelination along with increased accumulation of microglia and axonal damage in the corpus callosum, whereas all analyzed parameters were significantly less distinct in mice fed with cuprizone-containing pellets at an equivalent concentration of cuprizone at week 5. Even a higher concentration of cuprizone in pellet formulation was less potent compared to do so. This study illustrates that the established protocol of cuprizone intoxication (i.e., mixed in ground rodent chow) is the gold standard method to achieve consistent and reproducible demyelination. Why cuprizone loses its effectiveness in pellet formulation needs to be addressed in subsequent studies.



Similar content being viewed by others
References
Acs P, Komoly S (2012) Selective ultrastructural vulnerability in the cuprizone-induced experimental demyelination. Ideggyogyaszati szemle 65:266–270
Acs P et al (2009) 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia 57:807–814. doi:10.1002/glia.20806
Arnold S, Beyer C (2009) Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem 110:1–11. doi:10.1111/j.1471-4159.2009.06133.x
Biancotti JC, Kumar S, de Vellis J (2008) Activation of inflammatory response by a combination of growth factors in cuprizone-induced demyelinated brain leads to myelin repair. Neurochem Res 33:2615–2628. doi:10.1007/s11064-008-9792-8
Binder MD et al (2008) Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. J Neurosci: Off J Soc Neurosci 28:5195–5206. doi:10.1523/jneurosci.1180-08.2008
Blakemore WF (1972) Observations on oligodendrocyte degeneration, the resolution of status spongiosus and remyelination in cuprizone intoxication in mice. J Neurocytol 1:413–426
Carey EM, Freeman NM (1983) Biochemical changes in cuprizone-induced spongiform encephalopathy. I. Changes in the activities of 2′,3′-cyclic nucleotide 3′-phosphohydrolase, oligodendroglial ceramide galactosyl transferase, and the hydrolysis of the alkenyl group of alkenyl, acyl-glycerophospholipids by plasmalogenase in different regions of the brain. Neurochem Res 8:1029–1044
Chedid A, Jao W, Port J (1980) Megamitochondria in hepatic and renal disease. Am J Gastroenterol 73:319–324
Chen Z et al (2015) Cuprizone does not induce CNS demyelination in nonhuman primates. Ann Clin Transl Neurol 2:208–213. doi:10.1002/acn3.159
Dupree JL, Mason JL, Marcus JR, Stull M, Levinson R, Matsushima GK, Popko B (2004) Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol 1:179–192. doi:10.1017/S1740925X04000304
Fjaer S, Bo L, Lundervold A, Myhr KM, Pavlin T, Torkildsen O, Wergeland S (2013) Deep gray matter demyelination detected by magnetization transfer ratio in the cuprizone model. PLoS One 8:e84162. doi:10.1371/journal.pone.0084162
Franco-Pons N, Torrente M, Colomina MT, Vilella E (2007) Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett 169:205–213. doi:10.1016/j.toxlet.2007.01.010
Ghaiad HR, Nooh MM, El-Sawalhi MM, Shaheen AA (2016) Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: biochemical and histological study. Mol Neurobiol. doi:10.1007/s12035-016-9891-5
Goldberg J, Clarner T, Beyer C, Kipp M (2015) Anatomical distribution of cuprizone-induced lesions in C57BL6 mice. J Mol Neurosci. doi:10.1007/s12031-015-0595-5
Groebe A, Clarner T, Baumgartner W, Dang J, Beyer C, Kipp M (2009) Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum (London, England) 8:163–174. doi:10.1007/s12311-009-0099-3
Grosse-Veldmann R, Becker B, Amor S, van der Valk P, Beyer C, Kipp M (2015) Lesion expansion in experimental demyelination animal models and multiple sclerosis lesions. Mol Neurobiol. doi:10.1007/s12035-015-9420-y
Gudi V, Gingele S, Skripuletz T, Stangel M (2014) Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned. Front Cell Neurosci 8:73. doi:10.3389/fncel.2014.00073
Hagemeyer N et al (2012) Erythropoietin attenuates neurological and histological consequences of toxic demyelination in mice. Molecular Medicine (Cambridge, Mass) 18:628–635. doi:10.2119/molmed.2011.00457
Hanzlikova V, Schiaffino S (1977) Mitochondrial changes in ischemic skeletal muscle. J Ultrastruct Res 60:121–133
Heckers S, Held N, Kronenberg J, Skripuletz T, Bleich A, Gudi V, Stangel M (2017) Investigation of cuprizone inactivation by temperature. Neurotox Res. doi:10.1007/s12640-017-9704-2
Hibbits N, Yoshino J, Le TQ, Armstrong RC (2012) Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN neuro 4:393–408. doi:10.1042/an20120062
Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK (1998) Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol 92:38–49
Hoflich KM, Beyer C, Clarner T, Schmitz C, Nyamoya S, Kipp M, Hochstrasser T (2016) Acute axonal damage in three different murine models of multiple sclerosis: a comparative approach. Brain Res 1650:125–133. doi:10.1016/j.brainres.2016.08.048
Hoppel CL, Tandler B (1973) Biochemical effects of cuprizone on mouse liver and heart mitochondria. Biochem Pharmacol 22:2311–2318
Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S (1996) A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862. doi:10.1006/bbrc.1996.1112
Kipp M (2016) Remyelination strategies in multiple sclerosis: a critical reflection. Expert Rev Neurother 16:1–3. doi:10.1586/14737175.2016.1116387
Kipp M, Nyamoya S, Hochstrasser T, Amor S (2016) Multiple sclerosis animal models: a clinical and histopathological perspective. Brain pathology (Zurich, Switzerland). doi:10.1111/bpa.12454
Lewis RJ (2008) Hazardous chemicals desk reference vol 6th Ed. John Wiley & Sons, Inc., Hoboken
Lindner M, Fokuhl J, Linsmeier F, Trebst C, Stangel M (2009) Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci Lett 453:120–125. doi:10.1016/j.neulet.2009.02.004
Ludwin SK (1978) Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Laboratory investigation; a Journal of Technical Methods and Pathology 39:597–612
Matsushima GK, Morell P (2001) The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathology (Zurich, Switzerland) 11:107–116
Morell P, Barrett CV, Mason JL, Toews AD, Hostettler JD, Knapp GW, Matsushima GK (1998) Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci 12:220–227. doi:10.1006/mcne.1998.0715
Porta EA, Bergman BJ, Stein AA (1965) Acute alcoholic hepatitis. Am J Pathol 46:657–689
Reddy PH, Reddy TP (2011) Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res 8:393–409
Sachs HH, Bercury KK, Popescu DC, Narayanan SP, Macklin WB (2014) A new model of cuprizone-mediated demyelination/remyelination. ASN neuro 6. doi:10.1177/1759091414551955
Scheld M et al (2016) Neurodegeneration triggers peripheral immune cell recruitment into the forebrain. J Neurosci 36:1410–1415. doi:10.1523/jneurosci.2456-15.2016
Schmidt T, Awad H, Slowik A, Beyer C, Kipp M, Clarner T (2013) Regional heterogeneity of cuprizone-induced demyelination: topographical aspects of the midline of the corpus callosum. J Mol Neurosci: MN 49:80–88. doi:10.1007/s12031-012-9896-0
Sidman RL, Angevine JB, Pierce ET (1971) Atlas of the mouse brain and spinal cord. Harvard University Press Cambridge, Cambridge
Skripuletz T et al (2010) Cerebellar cortical demyelination in the murine cuprizone model. Brain Pathology (Zurich, Switzerland) 20:301–312. doi:10.1111/j.1750-3639.2009.00271.x
Slowik A, Schmidt T, Beyer C, Amor S, Clarner T, Kipp M (2015) The sphingosine 1-phosphate receptor agonist FTY720 is neuroprotective after cuprizone-induced CNS demyelination. Br J Pharmacol 172:80–92. doi:10.1111/bph.12938
Soundarapandian MM, Selvaraj V, Lo UG, Golub MS, Feldman DH, Pleasure DE, Deng W (2011) Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci Rep 1:2. doi:10.1038/srep00002
Steelman AJ, Thompson JP, Li J (2012) Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci Res 72:32–42. doi:10.1016/j.neures.2011.10.002
Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ (2003) Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol 13:329–339
Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G (2001) Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull 55:175–185
Suzuki K, Kikkawa Y (1969) Status spongiosus of CNS and hepatic changes induced by cuprizone (biscyclohexanone oxalyldihydrazone). Am J Pathol 54:307–325
Tandler B, Hoppel CL (1973) Division of giant mitochondria during recovery from cuprizone intoxication. J Cell Biol 56:266–272
Tejedor LS, Wostradowski T, Gingele S, Skripuletz T, Gudi V, Stangel M (2017) The effect of stereotactic injections on demyelination and remyelination: a study in the cuprizone model. J Mol Neurosci. doi:10.1007/s12031-017-0888-y
Torkildsen O, Brunborg LA, Myhr KM, Bo L (2008) The cuprizone model for demyelination. Acta Neurol Scand Suppl 188:72–76. doi:10.1111/j.1600-0404.2008.01036.x
Vakilzadeh G et al (2015) Protective effect of a cAMP analogue on behavioral deficits and neuropathological changes in cuprizone model of demyelination. Mol Neurobiol 52:130–141. doi:10.1007/s12035-014-8857-8
Vakilzadeh G, Khodagholi F, Ghadiri T, Ghaemi A, Noorbakhsh F, Sharifzadeh M, Gorji A (2016) The effect of melatonin on behavioral, molecular, and histopathological changes in cuprizone model of demyelination. Mol Neurobiol 53:4675–4684. doi:10.1007/s12035-015-9404-y
Venturini G (1973) Enzymic activities and sodium, potassium and copper concentrations in mouse brain and liver after cuprizone treatment in vivo. J Neurochem 21:1147–1151
Wagenknecht N, Becker B, Scheld M, Beyer C, Clarner T, Hochstrasser T, Kipp M (2016) Thalamus degeneration and inflammation in two distinct multiple sclerosis animal models. J Mol Neurosci: MN. doi:10.1007/s12031-016-0790-z
Wakabayashi T (2002) Megamitochondria formation—physiology and pathology. J Cell Mol Med 6:497–538
Werner SR, Saha JK, Broderick CL, Zhen EY, Higgs RE, Duffin KL, Smith RC (2010) Proteomic analysis of demyelinated and remyelinating brain tissue following dietary cuprizone administration. J Mol Neurosci 42:210–225. doi:10.1007/s12031-010-9354-9
Zatta P et al (2005) Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell Mol Life Sci: CMLS 62:1502–1513. doi:10.1007/s00018-005-5073-8
Zeldich E, Chen CD, Avila R, Medicetty S, Abraham CR (2015) The anti-aging protein klotho enhances remyelination following cuprizone-induced demyelination. J Mol Neurosci 57:185–196. doi:10.1007/s12031-015-0598-2
Zendedel A, Beyer C, Kipp M (2013) Cuprizone-induced demyelination as a tool to study remyelination and axonal protection. J Mol Neurosci 51:567–572. doi:10.1007/s12031-013-0026-4
Acknowledgments
We would like to thank S. Wübbel, A. Baltruschat, B. Aschauer, B. Mosler, and S. Tost for the excellent technical assistance. The present work was supported by the Doktor Robert Pfleger–Stiftung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were approved by the Review Board for the Care of Animal Subjects of the district government of Upper Bavaria (55.2-1-54-2532-73-15).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hochstrasser, T., Exner, G.L., Nyamoya, S. et al. Cuprizone-Containing Pellets Are Less Potent to Induce Consistent Demyelination in the Corpus Callosum of C57BL/6 Mice. J Mol Neurosci 61, 617–624 (2017). https://doi.org/10.1007/s12031-017-0903-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0903-3