Abstract
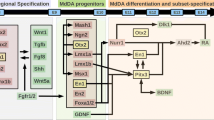
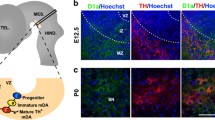
Mesencephalic and diencephalic dopaminergic (mdDA) progenitors generate two major groups of neurons corresponding to the A9 neurons of the substantia nigra pars compacta (SNpc) and the A10 neurons of the ventral tegmental area (VTA). MdDA neurons control motor, sensorimotor and motivated behaviour and their degeneration or abnormal functioning is associated to Parkinson’s disease and psychiatric disorders. Although relevant advances have been made, the molecular basis controlling identity, survival and vulnerability to neurodegeneration of SNpc and VTA neurons remains poorly understood. Here, we will review recent findings on the role exerted by the transcription factor Otx2 in adult mdDA neurons. Otx2 expression is restricted to a relevant fraction of VTA neurons and absent in the SNpc. In particular, Otx2 is prevalently excluded from neurons of the dorsal–lateral VTA, which expressed Girk2 and high level of the dopamine transporter (Dat). Loss and gain of function mouse models revealed that Otx2 controls neuron subtype identity by antagonizing molecular and functional features of the dorsal–lateral VTA such as Girk2 and Dat expression as well as vulnerability to the parkinsonian MPTP toxin. Furthermore, when ectopically expressed in the SNpc, Otx2 suppresses Dat expression and confers efficient neuroprotection to MPTP toxicity by suppressing efficient DA uptake.


Similar content being viewed by others
References
Dahlström A, Fuxe K (1964) Localization of monoamines in the lower brain stem. Experientia 20:398–399
Hökfelt T, Matensson A, Björklund S, Kleinau S, Goldstein M (1984) Distributional maps of tyrosine hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy: classical transmitters in the CNS. 2:227–379. Elsevier, Amsterdam
Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Biörklund A, Hökfelt (eds) Handbook of chemical neuroanatomy: classical transmitters in the CNS. 2:55–121. Elsevier, Amsterdam
Jellinger KA (2001) The pathology of Parkinson’s disease. Adv Neurol 86:55–72
Egan MF, Weinberger DR (1997) Neurobiology of schizophrenia. Curr Opin Neurobiol 7:701–707
Klockgether T (2004) Parkinson’s disease: clinical aspects. Cell Tissue Res 318:115–120
von Bohlen und Halbach O, Schober A, Krieglstein K (2004) Genes, proteins, and neurotoxins involved in Parkinson’s disease. Prog Neurobiol 73:151–177
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311
Isacson O (1993) On neuronal health. Trends Neurosci 16:306–308
Smidt MP, Burbach JP (2007) How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci 8:21–32
Smits SM, Burbach JP, Smidt MP (2006) Developmental origin and fate of meso-diencephalic dopamine neurons. Prog Neurobiol 78:1–16
Simeone A (2005) Genetic control of dopaminergic neuron differentiation. Trends Neurosci 28:62–65, discussion 65–66
Prakash N, Wurst W (2006) Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci 63:187–206
Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA 95:4013–4018
Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC (1998) A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet 19:167–170
Simeone A, Puelles E, Acampora D (2002) The Otx family. Curr Opin Genet Dev 12:409–415
Smidt MP, van Schaick HS, Lanctôt C, Tremblay JJ, Cox JJ, van der Kleij AA, Wolterink G, Drouin J, Burbach JP (1997) A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci USA 94:13305–13310
Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP (2000) A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci 3:337–341
Smidt MP, Smits SM, Bouwmeester H, Hamers FP, van der Linden AJ, Hellemons AJ, Graw J, Burbach JP (2004) Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development 131:1145–1155
Kele J, Simplicio N, Ferri AL, Mira H, Guillemot F, Arenas E, Ang SL (2006) Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 133:495–505
Ferri AL, Lin W, Mavromatakis YE, Wang JC, Sasaki H, Whitsett JA, Ang SL (2007) Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134:2761–2769
Andersson E, Jensen JB, Parmar M, Guillemot F, Björklund A (2006) Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development 133:507–516
Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J (2006) Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124:393–405
Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T (2007) Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134:3213–3225
McCaffery P, Dräger UC (1994) High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci USA 91:7772–7776
Jacobs FM, Smits SM, Noorlander CW, von Oerthel L, van der Linden AJ, Burbach JP, Smidt MP (2007) Retinoic acid counteracts developmental defects in the substantia nigra caused by Pitx3 deficiency. Development 134:2673–84
van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J (2003) Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130:2535–2542
Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP (2003) Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA 100:4245–4250
Simon HH, Saueressig H, Wurst W, Goulding MD, O’Leary DD (2001) Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J Neurosci 21:3126–3234
Sonnier L, Le Pen G, Hartmann A, Bizot JC, Trovero F, Krebs MO, Prochiantz A (2007) Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J Neurosci 27:1063–1071
Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276:248–250
Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E (2003) Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci U S A 100:12747–12752
Puelles E, Acampora D, Lacroix E, Signore M, Annino A, Tuorto F, Filosa S, Corte G, Wurst W, Ang SL, Simeone A (2003) Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat Neurosci 6:453–460
Puelles E, Annino A, Tuorto F, Usiello A, Acampora D, Czerny T, Brodski C, Ang SL, Wurst W, Simeone A (2004) Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 131:2037–2048
Omodei D, Acampora D, Mancuso P, Prakash N, Di Giovannantonio LG, Wurst W, Simeone A (2008) Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development 135:3459–70
Prakash N, Brodski C, Naserke T, Puelles E, Gogoi R, Hall A, Panhuysen M, Echevarria D, Sussel L, Weisenhorn DM, Martinez S, Arenas E, Simeone A, Wurst W (2006) A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development 133:89–98
Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, Abreu P, Giráldez T, Castro-Hernández J, Salas-Hernández J, Lanciego JL, Rodríguez M, González-Hernández T (2009) Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol Dis 36:494–508
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:35–172
Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O (2005) Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet 14:1709–25
Greene JG, Dingledine R, Greenamyre JT (2005) Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis 18:19–31
Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y, Raisman-Vozari R (1997) An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience 8:345–357
Liang CL, Sinton CM, German DC (1996) Midbrain dopaminergic neurons in the mouse: co-localization with Calbindin-D28K and calretinin. Neuroscience 75:523–33
Liang CL, Sinton CM, Sonsalla PK, German DC (1996) Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration 5:313–8
Schein JC, Hunter DD, Roffler-Tarlov S (1998) Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol 204:432–50
Thompson L, Barraud P, Andersson E, Kirik D, Björklund A (2005) Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci 25:6467–6477
Roffler-Tarlov S, Martin B, Graybiel AM, Kauer JS (1996) Cell death in the midbrain of the murine mutation weaver. J Neurosi 16:1819–26
Di Salvio M, Di Giovannantonio LG, Omodei D, Acampora D, Simeone A (2010) Otx2 expression is restricted to dopaminergic neurons of the ventral tegmental area in the adult brain. Int J Dev Biol 54:939–945
Di Salvio M, Di Giovannantonio LG, Acampora D, Prosperi R, Omodei D, Prakash N, Wurst W, Simeone A (2010) Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci. doi:10.1038/nn.2661
Wallén A, Zetterström RH, Solomin L, Arvidsson M, Olson L, Perlmann T (1999) Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp Cell Res 253:737–46
Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC (2006) Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 4:383–390
Kittappa R, Chang WW, Awatramani RB, McKay RD (2007) The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol 5:325
Heimbucher T, Murko C, Bajoghli B, Aghaallaei N, Huber A, Stebegg R, Eberhard D, Fink M, Simeone A, Czerny T (2007) Gbx2 and Otx2 interact with the WD40 domain of Groucho/Tle corepressors. Mol Cell Biol 27:340–51
Agoston Z, Schulte D (2009) Meis2 competes with the Groucho co-repressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrain-hindbrain boundary organizer. Development 136:3311–3322
Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 279:21012–21020
Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG (2003) Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem 278:2731–2739
Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog Neurobiol 77:128–138
Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T, Metzger D, Chambon P, Lindqvist E, Larsson NG, Olson L, Björklund A, Ichinose H, Perlmann T (2009) Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci 29:15923–15932
Pifl C, Giros B, Caron MG (1993) Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J Neurosci 13:4246–4253
Bezard E, Gross CE, Fournier MC, Dovero S, Bloch B, Jaber M (1999) Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp Neurol 155:268–273
Acknowledgments
The experiments and findings here discussed have been supported by the FP7 for the project mdDA NEURODEV (222999), the FP6 project for the EUTRACC Integrated Project (LSHG-CT-2007-037445), the “Stem Cell Project” of Fondazione Roma, and the Italian Association for Cancer Research (AIRC) and the MIUR for the PRIN project (20079ZLWYP_003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simeone, A., Di Salvio, M., Di Giovannantonio, L.G. et al. The Role of Otx2 in Adult Mesencephalic–Diencephalic Dopaminergic Neurons. Mol Neurobiol 43, 107–113 (2011). https://doi.org/10.1007/s12035-010-8148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-010-8148-y